A growing body of evidence suggest that obese individuals are under risk of renal parenchymal disorders when compared to nonobese counterparts. Microalbuminuria is the early marker of renal involvement. Although most of obese patients carries multiple risk factors for microalbuminuria, some obese individuals without risk factor may progress to microalbuminuria. The present study was performed to examine the role of ICAM-1 gene 1462A>G (K469E) polymorphism on microalbuminuria in obese subjects without diabetes mellitus, hypertension, hiperlipidemia and older age.
MethodsNinety eight obese and 96 nonobese individuals without a comorbidity enrolled into the study. Serum ICAM-1 level was measured by enzyme linked immunoabsorbent assay (ELISA) method. ICAM-1 gene 1462A>G (K469E) polymorphism was examined by restriction fragment length polymorphism-polymerase chain reaction (RFLP-PCR). Nepholometric method was used to examine urinary albumin loss, and microalbuminuria was measured by albumin to creatinine ratio.
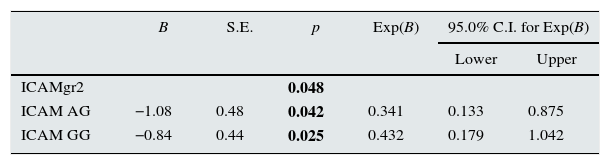
ResultsObese individuals had significantly higher microalbuminuria and proteinuria level compared to nonobese subjects (p: 0.043 and p: 0.011; respectively). GG genotype of ICAM-1 carriers have significantly higher microalbuminuria compared to individuals with AA or AG genotype carriers (p: 0.042). Serum ICAM-1 level was significantly correlated with creatinine and microalbuminuria (p: 0.002 and p: 0.03; respectively). Logistic regression analysis indicated a 7.39 fold increased risk of microalbuminuria in individuals with GG genotype of ICAM-1 gene 1462A>G (K469E) polymorphism.
ConclusionsGG genotype of ICAM-1 gene K469E polymorphism is associated with increased microalbuminuria in obese individuals without another metabolic risk factor.
Un conjunto de datos en aumento indica que los individuos obesos corren más riesgo de sufrir trastornos del parénquima renal si se los compara con sus homólogos no obesos. La oligoalbuminuria es un primer rasgo de afectación renal. Aunque la mayoría de los pacientes obesos presentan múltiples factores de riesgo de oligoalbuminuria, esta puede manifestarse en algunos individuos obesos sin factores de riesgo. El presente estudio se realizó para analizar el papel del polimorfismo 1462A>G (K469E) del gen ICAM-1 en la oligoalbuminuria de individuos obesos sin diabetes mellitus, hipertensión, hiperlipidemia ni vejez.
MétodosPara el estudio fueron reclutados 98 individuos obesos y 96 individuos no obesos sin comorbilidad. Se midió el nivel sérico de ICAM-1 mediante el ensayo de inmunoabsorción enzimática (ELISA). Se analizó el polimorfismo 1462A>G (K469E) del gen ICAM-1 por reacción en cadena de la polimerasay polimorfismo de longitud de los fragmentos de restricción (RFLP-PCR). El método nefolométrico se utilizó para analizar la pérdida urinaria de albúmina, y la oligoalbuminuria se midió con la tasa de albúmina/creatinina.
ResultadosLos individuos obesos presentaron unos niveles de oligoalbuminuria y proteinuria considerablemente más elevados que los individuos no obesos (p: 0,043 y p: 0,011, respectivamente). La oligoalbuminuria en los portadores del genotipo GG de ICAM-1 fue bastante mayor que la de los portadores del genotipo AA o AG (p: 0,042). El nivel sérico de ICAM-1 se correlacionó notablemente con la creatinina y la oligoalbuminuria (p: 0,002 y p: 0,03, respectivamente). El análisis de regresión logística mostró un riesgo 7,39 veces mayor de oligoalbuminuria en los individuos con el genotipo GG del polimorfismo 1462A>G (K469E) del gen ICAM-1.
ConclusionesEl genotipo GG del polimorfismo 1462A>G (K469E) del gen ICAM-1 se asocia con un aumento de la oligoalbuminuria en personas obesas sin otro factor de riesgo metabólico.
Obesity is becoming worldwide epidemic which accounts a risk factor for metabolic and hormonal disorders.1 Because insulin resistance is frequently seen, obese individuals are considered as candidates of diabetes mellitus (DM) and associated microvascular complications.2 Additionally, increased adipose tissue is also a source of subclinic inflammation which is closely related to endothelial damage.
Microalbuminuria (MA) is initial stage of diabetic nephropathy which is strongly correlated with endothelial injury.3 MA comprise an independent risk factor for coronary artery disease in both diabetic and nondiabetic population.4 Obese individuals even without overt DM and hypertension may exhibit microalbuminuria.5,6 Enhanced blood flow and glomerular filtration rate (GFR) are resulted with MA. An increasing number of evidence indicates low-grade inflammation as a cause of MA.7 Also obesity is a well-known risk factor for focal segmental glomerulosclerosis.8
One of the suggested mechanisms in the pathogenesis of microalbuminuria is overexpression of adhesion molecules including ICAM-1 which closely associated with severe inflammatory processes that usually resulted with tissue injury.9 Patients with obesity and diabetic nephropathy exhibit overexpression of ICAM-1.10,11 Experimental studies showed that hyperglycemia is associated with overexpression of ICAM-1 in renal glomerular tissue and renal endothelial injury.12 However, varying degree of renal injury among patients with normal ICAM-1 level or disassociation of ICAM-1 level with microalbuminuria may suggest the role of genetic mutations in ICAM-1 gene. As a marker of cell integrity and complexity, the effect of ICAM-1 on renal tissue is well-documented however lack of available data exist regarding to the role of ICAM-1 gene 1462A>G (K469E polymorphism on microalbuminuria in obese individuals.
Our aim is to examine the role of serum ICAM-1 level and ICAM-1 gene 1462A>G polymorphism on urine albumin loss in metabolically normal obese and nonobese individuals.
Material and methodsA total of 194 participants; 98 obese and 96 nonobese, older than 18 years without a history of systemic disorder that admitted to obesity outpatient service of Bagcilar Education and Research Hospital between February 2015 and June 2015 were enrolled. Height and weight of participants were measured in Tanita Body Composition Analyzer (Tanita Corporation of America, Illinois, USA). Body mass index (BMI) was calculated as weight/height2 (kg/m2). Body mass index (BMI) ≥30kg/m2 was defined as obesity.13 Exclusion criterias were secondary obesity, fasting glucose ≥100mg/dl, HbA1c ≥5.8, hypertension, hyperlipidemia, CVD, renal parenchymal disorder or urolitiazis.14 Hypertension was defined as systolic blood pressure ≥140mmHg and/or diastolic blood pressure ≥90mmHg, or receiving any antihypertensive agent. Renal parenchymal disorder or urolitiazis was diagnosed by ultrasonographic examination. National Cholesterol Education Programme (NCEP) was used to define hyperlipidemia (LDL-cholesterol>130mg/dl or trygliceride>200mg/dl).15 Ethics committee of Bagcilar Education and Research Hospital approved the study. Written informed consent was obtained from all participants. The study was performed in accordance with Declaration of Helsinki. Clinical Trials Registration number was 09.2014.0310. Registration date was 16/01/2015
Sample collection and analysisBlood samples were obtained after 8-h fasting period. Serum ICAM-1 concentration was analyzed by enzyme linked immunosorbent assay (ELISA) by commercially available kits (Invitrogen, Thermo Fisher Scientific, USA). Whole blood count was performed in blood samples that were taken into tubes with EDTA using an automatic blood counter (XE-5000; Sysmex Corp, Kobe, Japan). Biochemical variables including serum glucose, urea, creatinine, uric acid, aspartat aminotransferase (AST), alanine aminotransferase (ALT), lactate dehidrogenase (LDH), calcium, sodium, potassium, clor, total protein, albumin, total cholesterol, low density lipoprotein (LDL), high density lipoprotein (HDL), trigliceride, were analyzed by photometric method in Siemens Advia 1800 device (Siemens Healthcare Diagnostics, Kobe, Japan). Hormon parameters including HbA1c, insulin, C-peptide, TSH were analyzed by chemiluminescence immunoassay method in Siemens Advia Centaur device (XE-5000, Sysmex Corp., Kobe, Japan).
Urine analysis was performed by spectrophotometric method in Siemens Advia 1800 device (XE-5000, Sysmex Corp., Kobe, Japan). Urinary albumin was measured by immunoturbidimetric method in Cobas auto analyzer (Roche Diagnostics, Germany). Urinary creatinine was analyzed by Aeroset autoanalyzer (Abbott Laboratories Inc., Abbott Park, IL, USA). Urine albumin excretion rate exceeding 20μg/min (30mg/day); in the absence of uncontrolled hypertension or urinary tract infection, was defined as microalbuminuria.
DNA extractionTotal genomic DNA was extracted from peripheral blood leucocytes using a purification kit (Thermo Scientific GeneJET™ Whole Blood Genomic DNA Purification Kit) according to previously described.16
Genotyping analysisTo analyze ICAM-1 gene 1462A>G polymorphism (K469E; rs5498 polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was. PCR amplification was generated using the following oligonucleotide primers: 5′-GGAACCCATTGCCCGAGC-3′ and reverse 5′-GGTGAGGATTGCATTAGGTC-3′ (product of 223bp).
A PCR reaction was carried out in a 10μl reaction volume containing 1×PCR buffer, 2mM MgCl2, 0.2mM each deoxynucleotide triphosphate (dNTPs, Fermentas, St. Leon-Rot, Germany), 40ng of DNA, 0.2μM of each primer (Bio Basic Inc., Ontario, Canada), and 0.5 unit of Taq DNA Polymerase (Fermentas). The PCR conditions were: 3min of initial denaturation at 94°C, followed by 30 cycles at 95°C for 30s, 30s at 65°C for annealing, and 30s at 72°C for extension, followed by 5min at 72°C for final extension. The PCR products were 223bp.
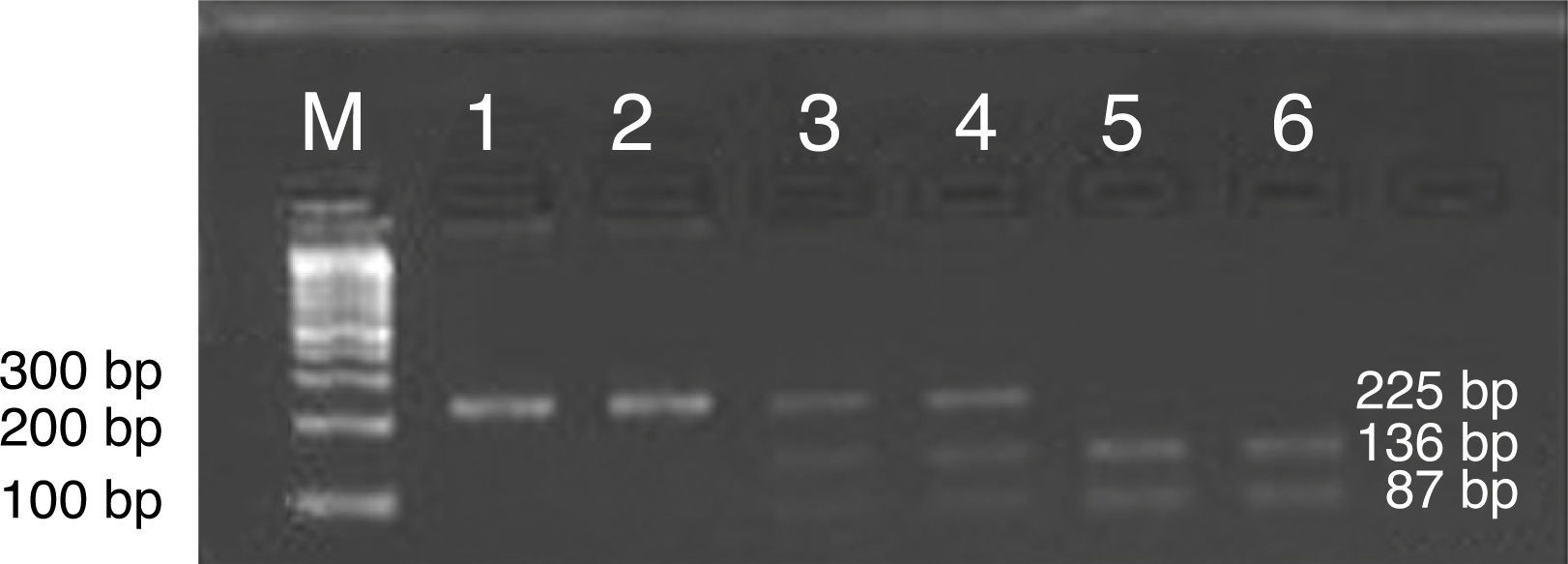
For the RFLP analysis, PCR-amplified products were digested with BanII for 2–5h at 37°C (New England Biolabs Inc., Hertfordshire, UK). The amplicon with the homozygous GG allele of ICAM was cleaved by BanII, yielding 136 and 87bp fragments, whereas the amplicon with the homozygous AA allele remained uncut, yielding a 223bp band. The amplicon with the heterozygous AG allele yielded three fragments of 223, 136 and 87bp length. The digested products were separated on 3% agarose gel along with a 100–1500bp DNA ladder (Bio Basic Inc.). Ethidium bromide-stained gels were visualized under UV light using the Alpha Imager System (Alpha Innotech, San Leandro, CA, USA) (Fig. 1).
Statistical analysisData were analyzed using the SPSS for Windows computer program (release 22.0; SPSS Inc. Chicago, IL, USA). All data expressed as the mean±SD. Differences between the study groups were analyzed by using Student's t-test. Quantitative data were analyzed by chi-square test. Pearson correlation analysis and Spearman's rho correlation analysis were used to evaluate the correlation of variables. The variables significant in univariate analysis were included to multivariate logistic regression analysis (only significant correlation coefficients are reported). A p value <0.05 was considered to be statistically significant.
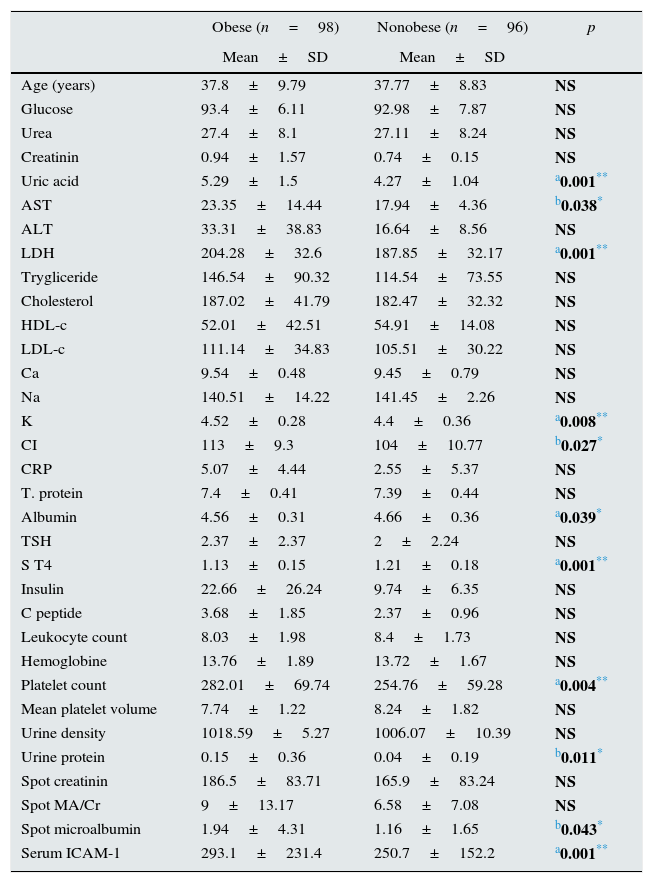
FindingsTable 1 shows demographic characteristics and laboratory parameters of obese and nonobese participants. Mean age of groups were similar however obese individuals had significantly higher uric acid, platelet count, microalbuminuria and proteinuria level (p: 0.001, p: 0.004, p: 0.043 and p: 0.011; respectively) (Table 1). Serum ICAM-1 level of obese individuals was significantly higher (p: 0.001).
Comparison of demographic characteristics and laboratory data between obese and nonobese individuals.
| Obese (n=98) | Nonobese (n=96) | p | |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age (years) | 37.8±9.79 | 37.77±8.83 | NS |
| Glucose | 93.4±6.11 | 92.98±7.87 | NS |
| Urea | 27.4±8.1 | 27.11±8.24 | NS |
| Creatinin | 0.94±1.57 | 0.74±0.15 | NS |
| Uric acid | 5.29±1.5 | 4.27±1.04 | a0.001** |
| AST | 23.35±14.44 | 17.94±4.36 | b0.038* |
| ALT | 33.31±38.83 | 16.64±8.56 | NS |
| LDH | 204.28±32.6 | 187.85±32.17 | a0.001** |
| Trygliceride | 146.54±90.32 | 114.54±73.55 | NS |
| Cholesterol | 187.02±41.79 | 182.47±32.32 | NS |
| HDL-c | 52.01±42.51 | 54.91±14.08 | NS |
| LDL-c | 111.14±34.83 | 105.51±30.22 | NS |
| Ca | 9.54±0.48 | 9.45±0.79 | NS |
| Na | 140.51±14.22 | 141.45±2.26 | NS |
| K | 4.52±0.28 | 4.4±0.36 | a0.008** |
| CI | 113±9.3 | 104±10.77 | b0.027* |
| CRP | 5.07±4.44 | 2.55±5.37 | NS |
| T. protein | 7.4±0.41 | 7.39±0.44 | NS |
| Albumin | 4.56±0.31 | 4.66±0.36 | a0.039* |
| TSH | 2.37±2.37 | 2±2.24 | NS |
| S T4 | 1.13±0.15 | 1.21±0.18 | a0.001** |
| Insulin | 22.66±26.24 | 9.74±6.35 | NS |
| C peptide | 3.68±1.85 | 2.37±0.96 | NS |
| Leukocyte count | 8.03±1.98 | 8.4±1.73 | NS |
| Hemoglobine | 13.76±1.89 | 13.72±1.67 | NS |
| Platelet count | 282.01±69.74 | 254.76±59.28 | a0.004** |
| Mean platelet volume | 7.74±1.22 | 8.24±1.82 | NS |
| Urine density | 1018.59±5.27 | 1006.07±10.39 | NS |
| Urine protein | 0.15±0.36 | 0.04±0.19 | b0.011* |
| Spot creatinin | 186.5±83.71 | 165.9±83.24 | NS |
| Spot MA/Cr | 9±13.17 | 6.58±7.08 | NS |
| Spot microalbumin | 1.94±4.31 | 1.16±1.65 | b0.043* |
| Serum ICAM-1 | 293.1±231.4 | 250.7±152.2 | a0.001** |
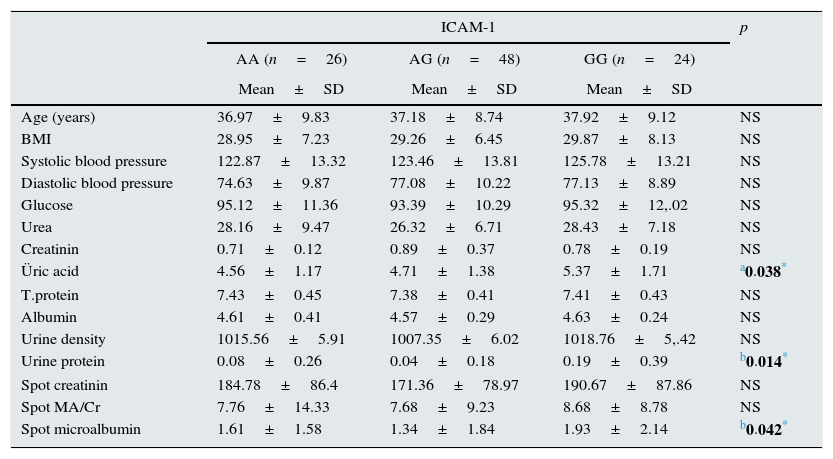
Table 2 demonstrates comparison of different genotypes of ICAM-1 gene with regard to demographic characteristics and laboratory parameters. Obese individuals with GG genotype had significantly higher uric acid, microalbuminuria and proteinuria when compared to individuals with AA or AG genotype (p: 0.038, p: 0.042 and p: 0.014; respectively) (Table 2).
Clinical and laboratory characteristics of obese group according to genotypes.
| ICAM-1 | p | |||
|---|---|---|---|---|
| AA (n=26) | AG (n=48) | GG (n=24) | ||
| Mean±SD | Mean±SD | Mean±SD | ||
| Age (years) | 36.97±9.83 | 37.18±8.74 | 37.92±9.12 | NS |
| BMI | 28.95±7.23 | 29.26±6.45 | 29.87±8.13 | NS |
| Systolic blood pressure | 122.87±13.32 | 123.46±13.81 | 125.78±13.21 | NS |
| Diastolic blood pressure | 74.63±9.87 | 77.08±10.22 | 77.13±8.89 | NS |
| Glucose | 95.12±11.36 | 93.39±10.29 | 95.32±12,.02 | NS |
| Urea | 28.16±9.47 | 26.32±6.71 | 28.43±7.18 | NS |
| Creatinin | 0.71±0.12 | 0.89±0.37 | 0.78±0.19 | NS |
| Üric acid | 4.56±1.17 | 4.71±1.38 | 5.37±1.71 | a0.038* |
| T.protein | 7.43±0.45 | 7.38±0.41 | 7.41±0.43 | NS |
| Albumin | 4.61±0.41 | 4.57±0.29 | 4.63±0.24 | NS |
| Urine density | 1015.56±5.91 | 1007.35±6.02 | 1018.76±5,.42 | NS |
| Urine protein | 0.08±0.26 | 0.04±0.18 | 0.19±0.39 | b0.014* |
| Spot creatinin | 184.78±86.4 | 171.36±78.97 | 190.67±87.86 | NS |
| Spot MA/Cr | 7.76±14.33 | 7.68±9.23 | 8.68±8.78 | NS |
| Spot microalbumin | 1.61±1.58 | 1.34±1.84 | 1.93±2.14 | b0.042* |
Male participants had significantly higher urea, creatinine, AST, ALT, LDH, triglyceride, total protein, albumin, FT4, hemoglobin and urine creatinine level while female participants had higher levels of body fat ratio and platelet count (p: 0.001, p: 0.001, p: 0.001, p: 0.001, p: 0.001, p: 0.023, p: 0.001, p: 0.001, p: 0.001, p: 0.001, p: 0.001, p: 0.001 and p: 0.002; respectively).
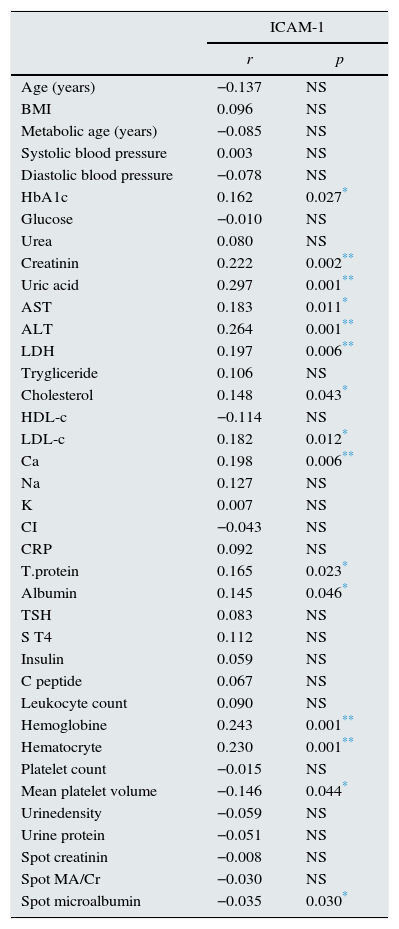
Correlation analysis indicated a significant correlation between serum ICAM-1 with HbA1c, creatinine, uric acid, AST, ALT, LDH, total cholesterol, LDL-c, albumin, hemoglobin and microalbuminuria (p: 0.027, p: 0.002, p: 0.001, p: 0.011, p: 0.001, p: 0.006, p: 0.043, p: 0.012, p: 0.046, p: 0.001 and p: 0.030; respectively) (Table 3). Association of creatinine with urea, uric acid, AST, ALT, LDH, albumin, TSH,FT4, WBC, hemoglobin, platelet count, urine creatinine and body fat mass were significant (p: 0.001, p: 0.001, p: 0.001, p: 0.001, p: 0.024, p: 0.004, p: 0.001, p: 0.001, p: 0.001 and p: 0.001; respectively).
Correlation of serum ICAM-1 with demographic and laboratory variables.
| ICAM-1 | ||
|---|---|---|
| r | p | |
| Age (years) | −0.137 | NS |
| BMI | 0.096 | NS |
| Metabolic age (years) | −0.085 | NS |
| Systolic blood pressure | 0.003 | NS |
| Diastolic blood pressure | −0.078 | NS |
| HbA1c | 0.162 | 0.027* |
| Glucose | −0.010 | NS |
| Urea | 0.080 | NS |
| Creatinin | 0.222 | 0.002** |
| Uric acid | 0.297 | 0.001** |
| AST | 0.183 | 0.011* |
| ALT | 0.264 | 0.001** |
| LDH | 0.197 | 0.006** |
| Trygliceride | 0.106 | NS |
| Cholesterol | 0.148 | 0.043* |
| HDL-c | −0.114 | NS |
| LDL-c | 0.182 | 0.012* |
| Ca | 0.198 | 0.006** |
| Na | 0.127 | NS |
| K | 0.007 | NS |
| CI | −0.043 | NS |
| CRP | 0.092 | NS |
| T.protein | 0.165 | 0.023* |
| Albumin | 0.145 | 0.046* |
| TSH | 0.083 | NS |
| S T4 | 0.112 | NS |
| Insulin | 0.059 | NS |
| C peptide | 0.067 | NS |
| Leukocyte count | 0.090 | NS |
| Hemoglobine | 0.243 | 0.001** |
| Hematocryte | 0.230 | 0.001** |
| Platelet count | −0.015 | NS |
| Mean platelet volume | −0.146 | 0.044* |
| Urinedensity | −0.059 | NS |
| Urine protein | −0.051 | NS |
| Spot creatinin | −0.008 | NS |
| Spot MA/Cr | −0.030 | NS |
| Spot microalbumin | −0.035 | 0.030* |
Spearman Rho test.
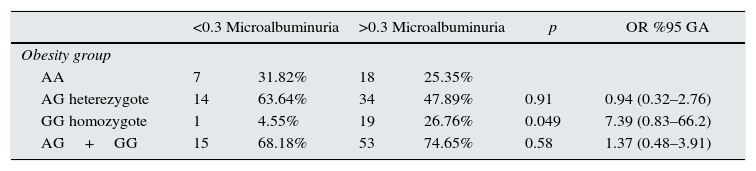
Multivariate analysis and Logistic regression analysis were performed to predict microalbuminuria risk in obese individuals with different genotypes of ICAM-1 gene single nucleotide polymorphism and determined that obese individuals with GG genotype of ICAM-1 gene 1462A>G polymorphism have 7.39 fold increased risk of microalbuminıria (p: 0.049) while the risk of microalbuminuria in individuals with AA or AG genotype were nonsignificant (Tables 4 and 5).
Logistic regression analysis to predict microalbuminuria in different genotypes of ICAM-1 gene K469E polymorphism.
| <0.3 Microalbuminuria | >0.3 Microalbuminuria | p | OR %95 GA | |||
|---|---|---|---|---|---|---|
| Obesity group | ||||||
| AA | 7 | 31.82% | 18 | 25.35% | ||
| AG heterezygote | 14 | 63.64% | 34 | 47.89% | 0.91 | 0.94 (0.32–2.76) |
| GG homozygote | 1 | 4.55% | 19 | 26.76% | 0.049 | 7.39 (0.83–66.2) |
| AG+GG | 15 | 68.18% | 53 | 74.65% | 0.58 | 1.37 (0.48–3.91) |
The present study indicated that obese individuals have worse metabolic control and evident microalbuminuria when compared to nonobese counterparts. Female individuals had significantly higher body fat ratio however metabolic deteriorament of male individuals was evident. GG genotype of ICAM-1 gene is associated with microalbuminuria and proteinuria. Finally, we determined a significant correlation between serum ICAM-1 level with microalbuminuria and metabolic control.
Endothelial dysfunction is the initial step of vascular changes which eventually progress to evident atherosclerosis (AS) and AS related complications.17 Endothelial injury and related disorders are five times more frequent and significantly seen at earlier ages among obese population.1 For more than a century ago, relation of obesity and AS has been known.18 Obesity is a well-known criteria of metabolic syndrome (MS) and accounts four times higher risk for atherosclerotic disorders.19 In a study including 9961 obese subjects, it has been shown that obesity is risk factor for microalbuminuria independent from diabetes and hypertension.20 In agreement with the literature, our obese population had higher blood pressure, lipid and uric acid levels, and excrete significantly higher amount of microalbumin and protein.
A growing body of evidence indicate that subclinical inflammation is evident in obese individuals which is closely related to AS and insulin resistance (IR).21 Inflammatory conditions are associated with increased endothelial activation and overexpression of adhesion molecules.22 Adhesion molecules regulates leukocyte migration across the endothelium and involved in integral part of inflammation.10 Obese individuals exhibit increased mRNA expression of ICAM-1 leading to infiltration of adipose tissue and other systems including kidneys by macrophages.23 Independent from fat distribution, total fat volume is major determinant of serum levels of adhesion molecules.24
Obese individuals or diabetic patients without well-controlled glycemia have increased ICAM-1 levels when compared to nonobese or diabetic patients with glycemic control.25,26 A well-known correlation has been established between diabetic complications including nephropathy and adhesion molecules.27 A number of studies determined that patients with ICAM-1 gene K469E polymorphism, the K allele are significantly more prone to endothelial injury and CVD, and may predict future cardiovascular events.28–30 There are controversial reports regarding to relation of ICAM-1 gene polymorphism and microvascular complications of DM. Sun et al. showed that ICAM-1 gene polymorphism has nonsignificant effect on diabetic retinopathy (DR) while Liu et all. determined an increased risk of DR in patients with ICAM-1 gene polymorphism31,32
Increased serum levels of adhesion molecules may considered as early markers of endothelial injury which is resulted with systemic vascular disorder including renal microvasculature.33,34 There is a positive correlation between serum ICAM-1 level and ICAM-1 gene polymorphism.35,36 It would be reasonable to expect microalbuminuria in obese individuals with overexpression of adhesion molecules because obese individuals have impaired insulin sensitivity, and also adhesion molecules destruct beta-cells leading to insulitis, hyperglycemia and hyperglycemia related nephrotoxicity that all may resulted with microalbuminuria. Adhesion molecules including ICAM-1 have been demonstrated in atherosclerotic lesions which is regressed by adiponectin, a adipocyte secretory protein decreased in obesity.37
The present study has some potential drawbacks. First, sample size of the study was relatively low. We included relatively young obese and nonobese individuals without diabetes, hypertension, hyperlipidemia, renal parenchymal or cardiovascular disorders to eliminate cumulative effect of these entities on microalbuminuria. Second, this study was conducted in Turkish population that limits us to generalize our results to other populations. Finally, evaluation of complex interaction of weight loss and serum ICAM-1 level may provide complementary data on microalbuminuria in obesity.
The importance/novelty of the study is that it is the first report that examined the role of ICAM-1 gene 1462A>G polymorphism on microalbuminuria in obesity, and established that GG genotype of ICAM-1 gene is an independent risk factor of microalbuminuria in obesity. There is a limited data on the genetic background of microalbuminuria in obese individuals. Understanding molecular and genetic mechanisms of microalbuminuria may help to identify obese patients under risk of nephropathy that is initially manifested with microalbuminuria.
Based on the fact that ICAM-1 is involved in endothelial injury and atherosclerosis, synthesis and genetic basis of this molecule may be therapeutic target for the complications of obesity. Further studies with large sample size are warranted to reach more precise conclusion.
Ethical disclosureThe study was performed in accordance with Helsinki Declaration.
Ethics Committee approval was obtained.
Informed consent was obtained from all participants.
Conflict of interestsAuthors declare that there is no conflict interest.