Clinical practice guidelines (CPG) are one of the most commonly used tools to ensure that at any given time the best available evidence is transferred into clinical practice. Unlike expert consensus documents, they are a set of recommendations based on a systematic review and critical reading of all the available evidence on a particular subject. The ultimate aims of CPG are to facilitate decision-making for the healthcare professional, optimise patient care and reduce variability in clinical practice.
The utility of CPG for improving clinical outcomes is widely recognised, although they have both intrinsic and extrinsic limitations.1 The methodology problems have largely been corrected thanks to the periodic recommendations of the AGREE project (http://www.agreetrust.org/agree-research-projects/) and, at a more local level, the recommendations of the Spanish National Health Service through the Healthcare Guide portal (http://portal.guiasalud.es).
Probably one of the main limitations, especially in nephrology, is the lack of sufficient evidence to be able to provide sound recommendations. Moreover, both the number and quality of controlled studies in nephrology are well below those in other specialist areas.2 If there are either no clinical trials or they are of poor quality, the weight of the evidence in CPG recommendations is diluted, and more prominence is given to the opinions of the “panel of experts” who draft the guidelines.
The current issue of Nefrologíahas published the abbreviated Spanish translation of the Clinical Practice Guideline on Diagnosis and Treatment of Hyponatraemia, developed by the European Society of Intensive Care Medicine (ESICM), the European Society of Endocrinology (ESE) and the European Renal Association – European Association for Dialysis and Transplantation (ERA-EDTA), represented by European Renal Best Practice (ERBP).3 This is a set of CPG put together according to the methodology proposed in AGREEII and published 2 years ago4 in an attempt to establish recommendations that can be easily accepted by the different specialists dealing with the diagnosis and treatment of hyponatraemia in their different clinical settings.
The European CPG came about as a result of the increased number of publications on hyponatraemia during the recent years, and the proliferation of documents on its management and treatment. This, in turn, is a direct consequence of the appearance in the market of vapants, aquaretic drugs for the treatment of hyponatraemia associated with SIADH (European Medicines Agency indication) and hypervolaemic hyponatraemia (FDA indication, added to that for SIADH). Any novel drug is an incentive for research on the drug itself and the diseases in which it is indicated. It is not surprising, therefore, that there has been a proliferation of different guidelines and documents on this disease. Among them, we would highlight the Spanish expert consensus on hyponatraemia,5 the international consensus on hyponatraemia6 and the European CPG summarised in this issue of Nefrología.4 The journal Nefrologíaalso discussed the importance of hyponatraemia in a supplement published in 2017.7 It should be noted that, in all these documents, most of the authors declared conflicts of interest with companies that market vaptans. Only for the European CPG funding was declared to be independent from the industry (funding was provided by the scientific societies involved).
In all these documents, the main problem is the lack of evidence to support the recommendations. The fact is that for disorders of the internal environment, there is a well-recognised lack of clinical trials evaluating the efficacy of different interventions for correcting them. Most of the guidelines are based on clinical experience and the translation of pathophysiology into clinical practice. As a result, the quality of the evidence of nearly all the recommendations in the European CPG on hyponatraemia is D (very low).
The combination of the lack of solid evidence and the need to give recommendations to other specialists besides nephrologists has stimulated nephrologist to generation of some statements about the European guidelines. Kidney specialists are very used to dealing with disorders of the body composition from basic concepts of renal physiology and pathophysiology. It is noticeable the absence of references to estimations of the sodium (Na), potassium (K), chlorine (Cl) and water balance, and the utility of electrolyte-free water clearance as tools to aid decision-making. The European CPG only mention symptoms, urinary osmolality (Osmu) and Nau if Osmu>100mOsm/kg. That is a very simplistic approach that can lead to misinterpretation. Thus, periodic monitoring of electrolyte-free water clearance by comparing Nap with the sum of urinary cations (Nau+Ku) allows us to estimate the capacity of the kidneys to excrete free water and anticipate the response to water restriction in the case of chronic non-hypovolaemic hyponatraemia and the tonicity of the fluid therapy administered.8 It also makes it possible to anticipate recovery by the kidneys of the ability to remove free water and prevent rapid correction of hyponatraemia. An example of the utility of these calculations is shown in Table 1. The clinical situations with the highest risk of rapid hyponatraemia correction are described in Table 2.
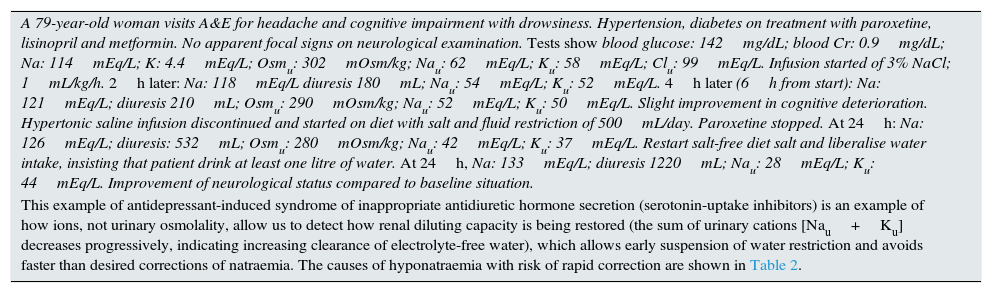
Example of the utility of urine electrolyte monitoring in the treatment of hyponatraemia.
| A 79-year-old woman visits A&E for headache and cognitive impairment with drowsiness. Hypertension, diabetes on treatment with paroxetine, lisinopril and metformin. No apparent focal signs on neurological examination. Tests show blood glucose: 142mg/dL; blood Cr: 0.9mg/dL; Na: 114mEq/L; K: 4.4mEq/L; Osmu: 302mOsm/kg; Nau: 62mEq/L; Ku: 58mEq/L; Clu: 99mEq/L. Infusion started of 3% NaCl; 1mL/kg/h. 2h later: Na: 118mEq/L diuresis 180mL; Nau: 54mEq/L; Ku: 52mEq/L. 4h later (6h from start): Na: 121mEq/L; diuresis 210mL; Osmu: 290mOsm/kg; Nau: 52mEq/L; Ku: 50mEq/L. Slight improvement in cognitive deterioration. Hypertonic saline infusion discontinued and started on diet with salt and fluid restriction of 500mL/day. Paroxetine stopped. At 24h: Na: 126mEq/L; diuresis: 532mL; Osmu: 280mOsm/kg; Nau: 42mEq/L; Ku: 37mEq/L. Restart salt-free diet salt and liberalise water intake, insisting that patient drink at least one litre of water. At 24h, Na: 133mEq/L; diuresis 1220mL; Nau: 28mEq/L; Ku: 44mEq/L. Improvement of neurological status compared to baseline situation. |
| This example of antidepressant-induced syndrome of inappropriate antidiuretic hormone secretion (serotonin-uptake inhibitors) is an example of how ions, not urinary osmolality, allow us to detect how renal diluting capacity is being restored (the sum of urinary cations [Nau+Ku] decreases progressively, indicating increasing clearance of electrolyte-free water), which allows early suspension of water restriction and avoids faster than desired corrections of natraemia. The causes of hyponatraemia with risk of rapid correction are shown in Table 2. |
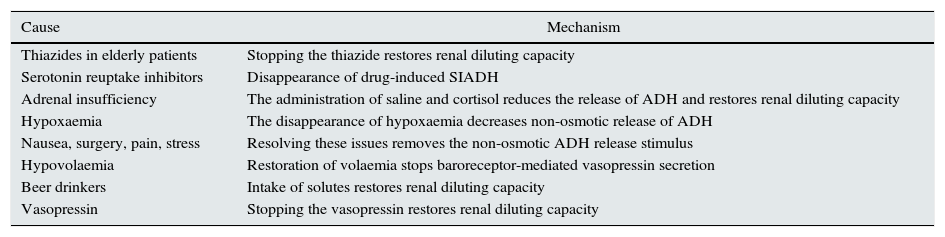
Causes of hyponatraemia with risk of rapid correction of the natraemia during treatment.
| Cause | Mechanism |
|---|---|
| Thiazides in elderly patients | Stopping the thiazide restores renal diluting capacity |
| Serotonin reuptake inhibitors | Disappearance of drug-induced SIADH |
| Adrenal insufficiency | The administration of saline and cortisol reduces the release of ADH and restores renal diluting capacity |
| Hypoxaemia | The disappearance of hypoxaemia decreases non-osmotic release of ADH |
| Nausea, surgery, pain, stress | Resolving these issues removes the non-osmotic ADH release stimulus |
| Hypovolaemia | Restoration of volaemia stops baroreceptor-mediated vasopressin secretion |
| Beer drinkers | Intake of solutes restores renal diluting capacity |
| Vasopressin | Stopping the vasopressin restores renal diluting capacity |
Nau is not always a good reflection of volaemia, however. In patients with volume depletion as a result of vomiting, the presence of organic anions such as HCO3− is accompanied by Na, so the Nau will be raised, but not the Clu.9
Only a holistic and individualised view of each patient, taking into account the contributions of active osmoles (including ions), both oral and parenteral, together with periodic monitoring of diuresis, blood ions in blood, and urine ions (Na, K, Cl) and osmolality will help provide clear guidance towards a diagnosis, optimise the treatment and anticipate possible complications resulting from inadequate correction of the hyponatraemia. Moreover, therapeutic decisions should be based on clinical and analytical progress, and not so much on an isolated determination of Nap; the consensus documents and the European CPG do not contemplate the analytical and biological variation inherent in measurements of Na.10 An actual natraemia of 121mEq/L may be reported by the laboratory in the interval 118–125mEq/L, which represents the critical difference (reference change value, RCV) of the Nap measurement with a 95% confidence interval, reinforcing the need to monitor and assess the hyponatraemic patient holistically.
Lastly, we would make two comments about the treatment. It is evident that hyponatraemia with moderate or severe symptoms benefits from treatment with hypertonic saline. A fixed therapeutic regimen is given using a solution (3% NaCl) which is not generally available in pharmacies, and it is not provided an easy “recipe” to prepare this hypertonic solution, as it is done in other documents.5,7 We personally prefer to provide this “recipe” (add 30mL of 20% NaCl to 250cc of 0.9% saline solution) and administer it according to the patient's estimated weight and the severity of their symptoms (0.5–2mL/kg/h). The second is the recommendation against the use of vaptans in the treatment of chronic normovolaemic hyponatraemia with the absence or only mild symptoms. It is surprising that the only therapeutic strategy (tolvaptan) to have proven useful in the treatment of normovolaemic and hypervolaemic chronic hyponatraemia in controlled, randomised studies is excluded as a valid option in the European CPG, based on the fact that it could increase the risk of pontine myelinolysis given the risk of rapid correction of natraemia, even though this complication was not detected in the clinical trials.11 However, they do recommend using oral urea, which has no clinical trials to support its utility and which, theoretically, could have the same risk of excessive correction as the vaptans.
CPG are highly useful tools for ensuring the best available evidence is transferred into clinical practice. The translated version of the CPG on hyponatraemia in this issue of Nefrología is undoubtedly a major effort to improve the therapeutic approach to hyponatraemia, and a reason for reflecting on the need to design studies in our speciality that not only improve and standardise the guidelines, but also the strength of the recommendations they contain. We encourage all nephrologists to read these European CPG with a critical and constructive eye. We hope that it serves as a stimulus for more in-depth investigation of the pathophysiology of hyponatraemia and the holistic management of this and other disorders of the internal environment.
Conflicts of interestR. Alcázar has received fees from Otsuka and Medical Nutrition for lectures. A. Tejedor has worked as a nephrology consultant for the Spanish Medicines Agency and the European Medicines Agency, and is a consultant for Otsuka.
Please cite this article as: Alcázar R, Tejedor, A. Guías ¿que no guían? Sobre hiponatremia. Nefrología. 2017;37:357–359.