We present a series of 52 patients with chronic kidney disease (CKD) on haemodialysis who presented SARS-CoV-2 infection diagnosed by nasopharyngeal swab polymerase chain reaction (PCR). The inclusion period was from 8 March 2020 until 21 February 2021, in which antibody testing for SARS-CoV-2 was performed during follow-up.
Mean age was 74.7 (±13.7) years, 80.7% were over 65; male sex predominated (73%), 7.6% were active smokers and 28.8% were former smokers. Of the main comorbidities, arterial hypertension (HTN) accounted for 96.1%, diabetes (DM) 69.2%, previous pulmonary disease 32.6%, coronary disease 25%, heart failure 23% and active cancer 3.8%. The most common chronic kidney disease (CKD) aetiologies were diabetic and vascular, followed by undetermined aetiology. A 15.8% had received a kidney transplant and 3.8% were on the transplat waiting list.
The most frequent clinical symptom was fever (75%), followed by cough (42.3%), dyspnoea (40.3%) and GI symptoms (diarrhoea 9.6%, nausea 5.7%). Mean lymphocyte count at admission was 1.23 (±2.0) × 10E9/L and C-reactive protein was 9.3 (±9.2) mg/dL.
The treatment received was lopinavir/ritonavir (57.6%), azythromycin (34.6%), hydroxychloroquine (30.7%), tocilizumab (9.6%), with neither remdesivir nor high steroid doses (Fig. 1).
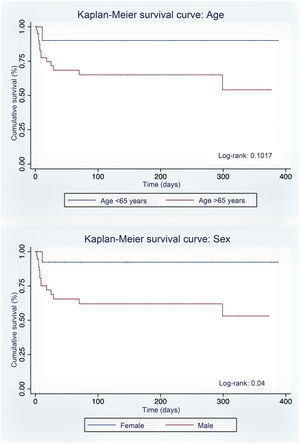
One of the 52 patients was managed as an outpatient. One patient was admitted to the Intensive Care Unit. Mortality was 31.3% (16 patients) due to SARS-CoV-2 pneumonia (31.3%), associated with being male and a smoker. There were no statistically significant differences in relation to the clinical and analytical variables (Table 1).
Characteristics of patients with SARS-CoV-2 infection.
| Variables | Survivors(n = 36) | Death(n = 16) | p |
|---|---|---|---|
| Age, years | 72.6 (15.6) | 79.3 (7.6) | 0.10 |
| Age >65 years, % | 63.4 | 36.5 | 0.10 |
| Sex m/f, % | 59.4/92.8 | 40.5/7.1 | 0.02 |
| Arterial hypertension, % | 67.3 | 32.6 | 0.32 |
| Diabetes mellitus, % | 72.2 | 27.7 | 0.39 |
| Coronary heart disease, % | 53.8 | 46.1 | 0.18 |
| Heart failure, % | 66.7 | 33.3 | 0.86 |
| Chronic pulmonary disease, % | 58.8 | 41.1 | 0.28 |
| Smoking: active, former/no, % | 47.3/84.6 | 52.6/15.3 | 0.003 |
| Previous kidney transplantation, % | 62.5 | 37.5 | 0.64 |
| Lymphocytes, x10E9/L | 0.89 (1.0) | 2.0 (3.3) | 0.08 |
| C-reactive protein, mg/dL | 8 (8.9) | 12.7 (10.0) | 0.14 |
M: male; F: female.
Of the survivors (36 patients, 68.6%), 19 patients (54.2%) were discharged home and 16 patients (45.6%) were transferred to a community health centre to continue their recovery. SARS-CoV-2 antibodies were determined in 33 patients using the (IgG Ab, Roche Cobas), 31 patients developed SARS-CoV-2 (91.6%) antibodies 39 days (21-104) after infection.
Chronic kidney disease patients on haemodialysis are known to present frequent infections plus a suboptimal response to vaccines, partly due to alterations in both innate and adaptive immunity1,2.
CKD is one of the risk factors that have been associated with greater mortality from SARS-CoV-2 infection3,4, since not only do these patients present immune system alterations, but further exposure to hospital centres renders them more vulnerable to contracting the infection.
Not a great deal is known about humoral response to SARS-CoV-2 infection in patients on haemodialysis. Sakhi et al. reported that seroconversion presented in 89% of the cases at a median 67 days post-diagnosis5, and while these data are similar to our population in terms of seroconversion, the development of antibodies in our cohort occurred earlier, at a median 39 days.
In conclusion, immunological response to SARS-CoV-2 infection in our patients on a haemodialysis programme was good, with seroconversion occurring in the majority of the patients. Nevertheless, this population’s future response to the SARS-CoV-2 vaccine is unknown.
Conflicts of interestMJ Soler declares that he/she has provided scientific consulting or given presentations with Mundipharma, Fresenius, Bayer, Novo Nordisk, Janssen, Boehringer, Eli Lilly, AstraZeneca and Esteve, which were not related to this work.
Nestor Toapanta, Zaira Castañeda, José Zúñiga, Natalia Ramos, María Azancot state that they have no conflict of interest.
FundingMJS currently holds research grants from the Fondo de Investigación Sanitaria-Feder – Instituto de Salud Carlos III (PI17/00257) and REDinREN (RD16/0009/0030).