Therapeutic plasma exchange (TPE) is indicated as a coadjuvant treatment in cases of severe acute kidney injury (AKI) secondary to vasculitis.1–7 Its benefit has been demonstrated both in the acute phase of the diseases and throughout the first year. It reduces the need of dialysis from 60% to 40%.3,5 Although its benefit has been shown in a range of diseases, therapeutic apheresis techniques have had limited application over the years. This could be explained, among other reasons, by the technical difficulty of the procedure, the rapid reoccurrence of some disease, and the limited number of controlled, randomised studies and meta-analyses demonstrating its usefulness; by contrast, there are a number of published articles with outdated views on its efficacy, safety and cost. Below, we discuss the results of a descriptive, longitudinal, single-centre study of a prospective database of patients with AKI secondary to vasculitis, treated with TPE in the Haematology and Haemotherapy Department of Miguel Servet University Hospital, Zaragoza. The severity of acute kidney injury is classified based on the Acute Kidney Injury Network (AKIN) classification and the Risk, Injury, Failure, Loss and End Stage Kidney Disease (RIFLE) criteria.
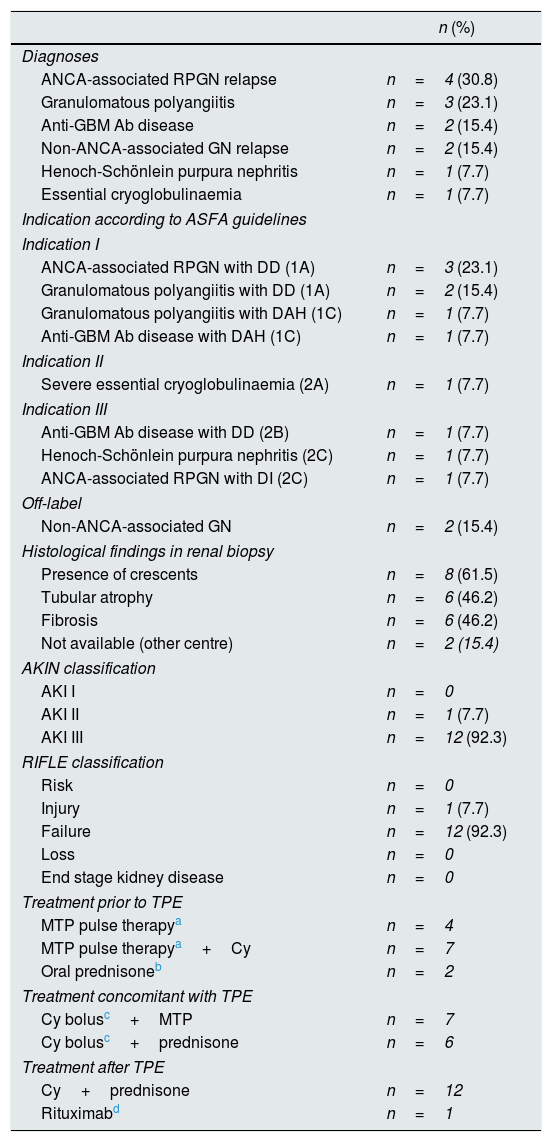
Thirteen cases were analysed, seven of which were women, with a median age of 67.7 years. 92.3% had high comorbidity (Charlson Index>4). More than 60% of the cases corresponded to indications type I or II of the guidelines of the American Society for Apheresis.2 All cases required support with dialysis, but this was only performed as an urgent procedure and before starting TPE in four cases (these four patients had serum creatinine values >6.8mg/dl). The rest of characteristics of the series are described in Table 1.
General characteristics of the series.
| n (%) | |
|---|---|
| Diagnoses | |
| ANCA-associated RPGN relapse | n=4 (30.8) |
| Granulomatous polyangiitis | n=3 (23.1) |
| Anti-GBM Ab disease | n=2 (15.4) |
| Non-ANCA-associated GN relapse | n=2 (15.4) |
| Henoch-Schönlein purpura nephritis | n=1 (7.7) |
| Essential cryoglobulinaemia | n=1 (7.7) |
| Indication according to ASFA guidelines | |
| Indication I | |
| ANCA-associated RPGN with DD (1A) | n=3 (23.1) |
| Granulomatous polyangiitis with DD (1A) | n=2 (15.4) |
| Granulomatous polyangiitis with DAH (1C) | n=1 (7.7) |
| Anti-GBM Ab disease with DAH (1C) | n=1 (7.7) |
| Indication II | |
| Severe essential cryoglobulinaemia (2A) | n=1 (7.7) |
| Indication III | |
| Anti-GBM Ab disease with DD (2B) | n=1 (7.7) |
| Henoch-Schönlein purpura nephritis (2C) | n=1 (7.7) |
| ANCA-associated RPGN with DI (2C) | n=1 (7.7) |
| Off-label | |
| Non-ANCA-associated GN | n=2 (15.4) |
| Histological findings in renal biopsy | |
| Presence of crescents | n=8 (61.5) |
| Tubular atrophy | n=6 (46.2) |
| Fibrosis | n=6 (46.2) |
| Not available (other centre) | n=2 (15.4) |
| AKIN classification | |
| AKI I | n=0 |
| AKI II | n=1 (7.7) |
| AKI III | n=12 (92.3) |
| RIFLE classification | |
| Risk | n=0 |
| Injury | n=1 (7.7) |
| Failure | n=12 (92.3) |
| Loss | n=0 |
| End stage kidney disease | n=0 |
| Treatment prior to TPE | |
| MTP pulse therapya | n=4 |
| MTP pulse therapya+Cy | n=7 |
| Oral prednisoneb | n=2 |
| Treatment concomitant with TPE | |
| Cy bolusc+MTP | n=7 |
| Cy bolusc+prednisone | n=6 |
| Treatment after TPE | |
| Cy+prednisone | n=12 |
| Rituximabd | n=1 |
Ab: antibodies; ANCA: antineutrophil cytoplasmic antibodies; AKIN: Acute Kidney Injury Network; Cy: cyclophosphamide; DD: dialysis dependence; OL: off-label; GN: glomerulonephritis; RPGN: rapidly progressive glomerulonephritis; DAH: diffuse alveolar haemorrhage; DI: dialysis independence; GBM: glomerular basement membrane; MTP: methylprednisolone; RIFLE: Risk, Injury, Failure, Loss and End Stage Kidney Disease; TPE: therapeutic plasma exchange.
The response rate after one month of TPE completion was 38.5%. Serum creatinine values below 5.8mg/dl were associated with better responses (p=0.032, RR: 0.16; 95% CI [0.02–1.03]), in keeping with a published bibliography of major series2–6.
The analysis revealed significant reductions in the values for serum creatinine (p=0.005), with the corresponding change in glomerular filtration rate (p=0.003) and proteinuria in a single urine sample (p=0.045). It was also noted a reduction in the values of platelets (p=0.022) and fibrinogen (p=0.037). However, these latter findings were not associated with haemorrhagic manifestations.
With regard to the plasma exchange technique, during the procedure we performed prophylaxis of hypocalcaemia with intravenous calcium gluconate, with repeated administration if symptoms appeared. At the end of the procedure, intravenous vitamin K was administered prophylactically with the aim of preventing dilutional coagulopathy. The devices used were continuous-flow blood cell separators (Cobe Spectra® or Optia Spectra®, Terumo) with adenine citrate dextrose solution A (ACD-A) as an anticoagulant. Central venous access were, Hickman (n=9), Shaldon (n=2) and femoral (n=2) catheters.
The median number of days between clinical suspicion and the initiation of TPE was 10 (0–28). Six sessions (4–17) per patient were carried out, with a volume of 3371 (2333–4759) ml exchanged, corresponding to 1–1.5 blood volumes. Replacement was performed with 5% albumin.
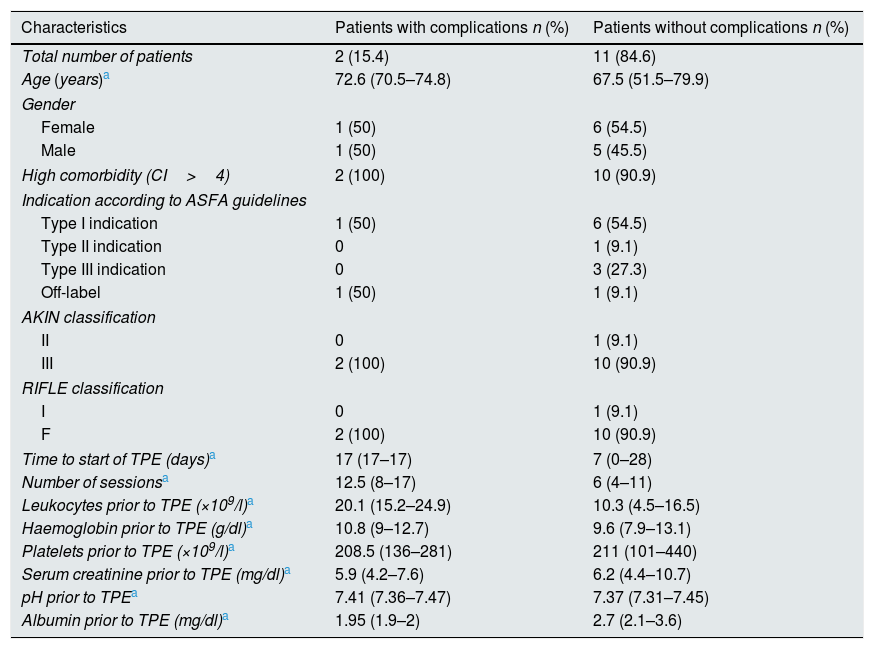
Of a total of 96 sessions, one (1.04%) had low access pressure and four (4.1%) had clinical complications (pruritus in three and fever in one), all of them corresponded to two patients (Table 2). None of these complications was severe and only one session had to be suspended due to the onset of fever. The patients who developed the more complications (clinical and technical) had a high leucocyte count prior starting TPE (p=0.04) and underwent more TPE sessions (p=0.010) (see Table 2). A higher rate of complications was also observed in TPEs performed before the year 2010 (28.6 vs 0%), without statistical significance.
Characteristics of patients with and without complications.
| Characteristics | Patients with complications n (%) | Patients without complications n (%) |
|---|---|---|
| Total number of patients | 2 (15.4) | 11 (84.6) |
| Age (years)a | 72.6 (70.5–74.8) | 67.5 (51.5–79.9) |
| Gender | ||
| Female | 1 (50) | 6 (54.5) |
| Male | 1 (50) | 5 (45.5) |
| High comorbidity (CI>4) | 2 (100) | 10 (90.9) |
| Indication according to ASFA guidelines | ||
| Type I indication | 1 (50) | 6 (54.5) |
| Type II indication | 0 | 1 (9.1) |
| Type III indication | 0 | 3 (27.3) |
| Off-label | 1 (50) | 1 (9.1) |
| AKIN classification | ||
| II | 0 | 1 (9.1) |
| III | 2 (100) | 10 (90.9) |
| RIFLE classification | ||
| I | 0 | 1 (9.1) |
| F | 2 (100) | 10 (90.9) |
| Time to start of TPE (days)a | 17 (17–17) | 7 (0–28) |
| Number of sessionsa | 12.5 (8–17) | 6 (4–11) |
| Leukocytes prior to TPE (×109/l)a | 20.1 (15.2–24.9) | 10.3 (4.5–16.5) |
| Haemoglobin prior to TPE (g/dl)a | 10.8 (9–12.7) | 9.6 (7.9–13.1) |
| Platelets prior to TPE (×109/l)a | 208.5 (136–281) | 211 (101–440) |
| Serum creatinine prior to TPE (mg/dl)a | 5.9 (4.2–7.6) | 6.2 (4.4–10.7) |
| pH prior to TPEa | 7.41 (7.36–7.47) | 7.37 (7.31–7.45) |
| Albumin prior to TPE (mg/dl)a | 1.95 (1.9–2) | 2.7 (2.1–3.6) |
AKIN: Acute Kidney Injury Network; ASFA: American Society for Apheresis; CI: Charlson Index; HD: haemodialysis; RIFLE: Risk, Injury, Failure, Loss and End Stage Kidney Disease; TPE: therapeutic plasma exchange.
It is reasonable to think that a higher number of sessions would lead to a higher number of adverse associated events. Even so, the overall rate of complications in our series was lower than that published in other studies.8–10 The finding of a higher initial leucocyte count in patients with more complications may be related to a higher starting dose of corticosteroids, although this assumption was not confirmed. The association of a higher initial leucocyte count with the development of complications needs to be evaluated in a series with more cases.
The fact that our rate of complications was less than that published by others leads us to conclude that TPE is a safe therapeutic strategy in our centre and encourages us to continue to perform it. We can postulate that the lack of complications may be attributed to the prophylaxis that is being used.
Please cite this article as: Parra Salinas IM, Arnaudas Casanova L, Blasco Forcén Á, González Rodríguez VP, García-Erce JA. Seguridad de los recambios plasmáticos terapéuticos en la lesión renal aguda secundaria a vasculitis. Nefrologia. 2018;38:567–570.