Associated renal and cardiac diseases have a high prevalence among the population in several clinical contexts: acute renal failure in the context of decompensated heart failure (HF), HF patients who develop chronic kidney disease (CKD) and patients with CKD who develop HF. In recent years, cardiorenal syndrome has been described as deteriorating kidney function in the context of HF. However, there are other clinical situations for which nephrologists can contribute their knowledge as a part of an integral treatment strategy, as is the case with refractory HF (RHF). All of these situations require an interdisciplinary cooperative effort between cardiologists and nephrologists with the aim of providing integral treatment. This article aims to review the role of the nephrologist in HF treatment, with an emphasis on the subgroup of patients with RHF and current evidence regarding the usefulness of peritoneal dialysis (PD) as a chronic coadjuvant treatment.
Las patologías renal y cardíaca asociadas son de alta prevalencia en la población en diferentes contextos clínicos: fracaso renal agudo en el contexto de insuficiencia cardíaca (IC) descompensada, pacientes con IC que desarrollan enfermedad renal crónica (ERC) o pacientes con ERC que desarrollan IC. En los últimos años se ha descrito el síndrome cardiorrenal (SCR) como el deterioro de la función renal en el contexto de IC. Sin embargo, existen otras situaciones clínicas en las que la Nefrología puede aportar su conocimiento como parte de la estrategia de tratamiento integral, como es el caso de la IC refractaria (ICR). Todas estas situaciones obligan a un trabajo conjunto interdisciplinario entre cardiólogos y nefrólogos con el fin de proporcionar un tratamiento integral. Este documento pretende hacer una revisión del papel del nefrólogo en el tratamiento de la IC haciendo hincapié en el subgrupo de pacientes con ICR y la evidencia actual de la utilidad de la diálisis peritoneal (DP) como tratamiento crónico coadyuvante.
INTRODUCTION
HF is a chronic progressive disorder with increasing incidence and prevalence due to the aging of the population and innovation in the treatment of patients with coronary and hypertensive pathologies, which are its principal causes. HF is one of the major causes of morbidity and mortality in the general population and it is responsible for high rates of hospitalisation and readmission;1 in spite of the advances made in its treatment, the overall mortality rate at 8 years reaches 80%.2 Among treatment strategies, diuretics are the most useful tools for eliminating excess fluids. However, heart failure refractory to conventional treatment (RHF) is an increasing pathology.1 HF is a public health problem and its worldwide prevalence is estimated at 23 million people. In the USA. it affects 2.3% of the population with increased incidence among the elderly.2,3 In Europe the prevalence in patients between 70 and 80 years of age is 10-20% and in Spain it is the main cause of hospitalisation in people over 65.4,5 Every year in the USA some 550,000 new cases are diagnosed; in 2003 it was the cause of one million hospital admissions and 57,000 deaths and its cost in 2005 was 27.9 billion dollars.2,3,6 RHF is not uncommon: it is estimated that in the USA between 50,000 and 200,000 patients have the condition and their survival rate is less than 50% at 6 months.7
Renal dysfunction is a common pathology in HF patients, with a prevalence of 36-50% and up to 25% of patients with CKD are diagnosed with HF, a figure that rises to 64% among patients who start dialysis.8-10 Furthermore, episodes of acute deterioration of renal function are often observed during the decompensation stages of HF.11
HEART FAILURE. CLASSIFICATION, PHYSIOPATHOLOGY AND PROGNOSIS FACTORS
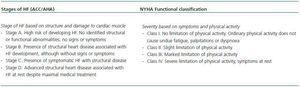
HF is a consequence of diverse diseases that affect cardiac tissue. There are two classification scales: that of the New York Heart Association (NYHA), which is based on symptoms and physical activity, and that designed by the American College of Cardiology/American Heart Association (ACC/AHA), based on structural abnormalities of the cardiac tissue (Table 1).4,12
Diverse haemodynamic, neurohormonal and immunological mechanisms participate in the physiopathology of HF that also affect renal function.2 The coexistance of HF and CKD is deleterious for both and it is associated with accelerated atherosclerosis, alteration in the regulation of intravascular volume and inadequate compensation of regulatory mechanisms, which finally leads to morbidity and mortality.13 Both share a common group of risk factors such as arterial hypertension (AH), diabetes mellitus (DM) and atherosclerosis.14 In HF the triggering factor is low cardiac output which leads to activation of the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system (SNS), alteration in the nitric oxide balance and release of antidiuretic hormone. All of these produce systemic vasoconstriction and the retention of sodium and water (antinatriuretic and antidiuretic effects). The latter causes progressive volume overload, contributes to the major symptoms referred by patients, unleashes the main cause of hospitalisation and has an effect on the progression of HF.15 Moreover, diverse inflammatory factors participate in the progressive damage to renal and cardiac tissue.9 Low cardiac output implies low renal perfusion and, in addition, the pharmacological treatment used in HF with diuretics and RAAS inhibitors may worsen renal function, especially during HF decompensation episodes or with previous renal dysfunction.6,16 Cardiorenal syndrome (CRS) appears, due to the worsening of HF and leads to renal failure (RF) causing hydric overload, resistance to the effect of diuretics and, finally, the development of HF refractory to treatment.1 There are two situations which favour renal resistance to the diuretic effect: the state of hypervolaemia secondary to the retention of liquids and sodium, and azotaemia secondary to renal hypoperfusion.15
TREATMENT OF HEART FAILURE
There are a number of alternatives for the treatment of HF. Figure 1 shows the objectives of the therapy. Standard therapy includes RAAS antagonists, beta-blockers, conventional diuretics and digitalic drugs. One of the fundamental pillars in the treatment of HF is the control of sodium and water balance. 80% of hospitalisations in HF cases are due to acute decompensations and the majority of these patients are admitted for hydric overload, while only 5% admissions are caused by low cardiac output.2 Many patients respond to standard treatment but the use of diuretics is not always effective. Mortality for this patient group is close to 75% in the following year.5 HF is considered terminal in those HF patients who are not candidates for heart transplant and who can only be offered palliative treatment. RHF symptoms are attributed particularly to the retention of sodium and water. Response to diuretics, which oscillates between 65 and 70%,17 is altered by many factors. Furthermore, some studies have shown an association between some classes of diuretics and mortality. The SOLVD study mentions an association between non potassium-sparing diuretics and an increased risk of hospitalisation and mortality.18 The challenge in the treatment of RHF patients to improve symptoms, reduce continuous hospitalisation and try to prolong survival has led to the development of new therapies to enhance myocardial contractility (inotropic and vasodilator drugs, resynchronization therapies, mechanical circulatory support, etc.). However, little progress has been made in hydric overload, the principal cause of symptoms in these patients. Refractoriness to diuretic treatment has led to the use of new strategies for the excretion and/or elimination of excess sodium and water and here is where nephrology can play a fundamental role.
ALTERNATIVE TREATMENTS TO IMPROVE THE EXCRETION OF SODIUM AND WATER
Among alternative treatments are the use of vasopressin receptor antagonists and ultrafiltration (UF) techniques.19,20
Vasopressin receptor antagonists (vaptans), whose pharmacological aim is to inhibit the V2 receptors of the medullary collecting duct segment, seem to be, in theory, an adequate strategy for inducing aqueous diuresis, reducing hydric overload and improving hyponatraemia. There are studies that have shown its usefulness in weight loss, hyponatraemia correction and mortality reduction.14 In contrast, in the EVEREST study of more than 4,000 recruited patients, which compared patients treated with tolvaptan in addition to standard treatment against patients receiving only standard treatment, no differences were observed in morbidity and mortality (improvement in functional class, reduction in hospital readmissions, reduction in mortality).21
Natriuretic peptides, in theory, induce vasodilation, natriuresis and RAAS suppression and SNS inhibition and among those assayed are recombinant brain natriuretic peptide (nesiritide) and ularitide (urodilatin). In the case of nesiritide, a meta-analysis has shown no theoretical benefit in the improvement of renal function and, in fact, it shows an increase in mortality.20
ULTRAFILTRATION TECHNIQUES
The national register of decompensated HF cites that 42% of patients are discharged without resolving their symptomatology and up to 70% with inadequate weight loss, which produces high rates of hospital readmission.9,22 This situation has led to the use of UF techniques, including peritoneal dialysis (PD), in acute and chronic situations to eliminate excess fluids. Extracorporeal UF was proposed by Silverstein in 1974 as a modification of the haemodialysis circuit. Since 1979 several studies have been published using this technique for the treatment of RHF patients.23 It has been proposed as an alternative treatment method in patients with acute decompensations and a rapid improvement of symptoms, reduction in the rate of readmission, reduction of pulmonary and peripheral oedema, improvement in functional class, restoration of the diuretic response and reduction of circulatory proinflammatory cytokines have been observed.2,24-26 However, other studies have shown the appearance of frequent episodes of hypotension, an increase in the need for diuretic treatment, lack of renal function recovery, anaemia and catheter-associated infections.2,24 The multicentric (UNLOAD) study, which included 200 acute decompensated HF patients, who were randomly selected to receive intravenous diuretics or early extracorporeal UF, reported a greater reduction in weight and loss of liquids as well as a lower incidence of readmission in the UF group, but no differences were observed in creatinine levels or mortality.27 There is a portable UF device on the market which can be used even at home with prior training.23 There is no doubt that extracorporeal UF, despite having no impact on mortality, is an additional tool in the treatment of patients with acute HF decompensation.
PERITONEAL DIALYSIS: AN ALTERNATIVE TREATMENT IN REFRACTORY HEART FAILURE
PD may be a treatment option for chronic RHF,6 with some advantages over haemodialysis (HD) in the treatment of these patients, such as greater preservation of residual renal function (RRF), continuous UF, enhanced haemodynamic stability, improved clearance of medium-sized molecules, sodium sieving with maintenance of normonatremia and less systemic inflammation.2 PD also favours the clearance of inflammatory molecules such as interleukin-1 (IL-1) and tumour necrosis factor (TNF) which play an important role in HF progression (myocardial depressors).2,28 PD, as a UF technique in RHF, was described by Schneierson29 in 1949 and has since been used in acute HF decompensations as well as in the ongoing treatment of chronic HF patients. The therapeutic modalities employed have varied from the initial intermittent PD (IPD) to continuous ambulatory PD (CAPD), APD and the use of new solutions such as icodextrin.2
Intermittent peritoneal dialysis
Many publications have reported that treatment with PD in acute decompensated HF patients has resulted in a reduction of pulmonary wedge pressure, restoration of the response to diuretics and even enhanced glomerular filtration.6 Mailloux, in a group of 15 patients, observed an improvement in the response to diuretics in 12 cases, weight reduction (5.2kg on average) and improved cardiac output.30 Shapira communicated his experience with 10 patients, observing weight reduction, improved clinical symptoms, increased diuresis with response to diuretics and normalization of sodium levels. These advantages were offset by the necessity for hospital treatment and the high rate of peritonitis by gramnegative bacteria he described.31 He has also described the appearance of arterial hypotension as a result of rapid UF.1 Due to the technical difficulties of acute IPD, extracorporeal UF methods are generally preferred as rescue therapy in hospitalised RHF patients who do not respond to diuretics.6,32
Chronic peritoneal dialysis (continuous ambulatory peritoneal dialysis and automated peritoneal dialysis)
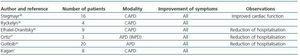
The role of chronic PD in the long term treatment of RHF patients has not been formally evaluated in large clinical studies and the data available up to now are, overall, from small series.6 The first published cases were RHF and non-terminal CKD patients treated with CAPD.33-37 The number of patients included was small and the duration of treatment varied between 5 and 24 months. The results were almost unanimous in improved clinical symptoms, functional class and reduction in the frequency of hospitalisation, although a high rate of peritonitis was also observed with no impact on mortality. The use of APD began in the 1990s. Since then, several reports have confirmed clinical improvement, a reduction in the expected mortality at one year, and improved cardiovascular parameters such as ejection fraction (EF) and pulmonary systolic blood pressure.2,6,7 Table 2 compiles the most important data from these studies in which, in addition, problems related to the technique, such as peritonitis, were significantly reduced.7,38-42 Gotloib’s prospective study includes the greatest number of patients and his main results are shown in Table 3.42 In this study, as in the others, patients were initially treated with extracorporeal UF to stabilize them during acute decompensation and they subsequently began chronic PD. This technique has also been used as a bridge for subsequent heart transplant treatment, and even in non-transplant candidates (terminal HF) as palliative treatment.43 There are currently no studies which compare PD treatment against the non-chronic use of a UF technique.
Opportunities related to icodextrin solution
Icodextrin is a high molecular weight glucose polymer that generates a prolonged oncotic pressure, which enhances UF during periods of extended stay. The use of icodextrin in PD solutions, compared to glucose solutions, has been associated with reduced generation of glucose degradation products (GDP), less intraperitoneal inflammation, a reduction in systemic glucose absorption, fewer carbohydrate and lipid metabolism alterations and greater UF with longer stays.6 These characteristics make it the ideal solution for the treatment of RHF, even using a single night exchange,1 although existing studies are still scarce. Bertoli used a nocturnal exchange with icodextrin in 2 non-uremic patients with NYHA function class III-IV. The daily UF obtained oscillated between 500 and 1,000ml and there was an improvement in functional class, an increase in EF (from 22 to 27% and from 25 to 50%) and better renal function, without the need for hospitalisation during the follow-up period.44 Díez’s Spanish group has published their experience with 5 patients with RHF and non-terminal CKD. In 3 cases they used a single exchange with icodextrin and in the remaining patients the classic CAPD treatment with nocturnal icodextrin. The follow-up period varied between 5 and 19 months and showed an improvement in the functional class of all patients and a reduction in hospitalisation rate (an average of 139 days/year before the therapy to 12 days/year after starting). One patient’s EF increased from 35 to 45% and two had a reduction in pulmonary systolic pressure, increasing glomerular filtration in one case.45 More recently, Basile has published his results in 4 patients treated with a single nocturnal icodextrin exchange and one with nocturnal icodextrin plus a 1.36% glucose exchange, with follow-up of between 11 and 43 months. In all cases there was an improvement in functional class and a reduction in hospitalisation, with increased urinary volume in one case. Patients did not develop peritonitis during the follow-up period.46 In spite of these promising results, only a few reviews of RHF therapy recommend the use of this kind of coadjuvant therapy in the chronic treatment of RHF patients.47
PROPOSAL FOR FUTURE STUDIES
In light of evidence-based medicine and in order to draw conclusions that help to answer some of those questions it would be appropriate to initiate several prospective studies in RHF and non-terminal CKD patients:
Comparative studies of patients treated with PD against standard treatment evaluating survival, quality of life, morbidity and cost-benefit advantages.
Comparative studies of patients treated with PD against standard treatment and observing the impact on baseline diseases: preservation of renal function and delayed HF progression.
Comparative studies of patients treated with different PD strategies: daily nocturnal icodextrin and daytime APD with icodextrin, to evaluate the repercussions on morbidity and mortality and complications associated with the technique.
Comparative studies of patients treated with PD against the use of new therapeutic strategies in the elimination of liquids (vaptans) and to observe the results of morbidity and mortality and public health costs.
KEY CONCEPTS
1. RHF is a frequent situation within the group of HF patients. This situation leads to an important deterioration in functional class with a high rate of hospitalisation due to frequent decompensations.
2. HF patients often have associated renal dysfunction that contributes to the refractoriness of diuretic treatment.
3. Different PD modalities (CAPD or APD) constitute an adjuvant therapy for the treatment of RHF patients, improving functional class and reducing the rate of hospitalisation.
4. PD solutions with icodextrin allow RHF patients to be treated with a single nocturnal exchange, which facilitates treatment and reduces complications associated with the technique.
Table 1. Heart failure classification
Figure 1. Treatment objectives in heart failure.
Table 2. Published studies in which chronic peritoneal dialysis is used in the treatment of congestive heart failure
Table 3. Characteristics of patients with refractory heart failure before and after APD treatment (three weekly sessions of 8 hours)42