The PROGRESER study is a multicentre, prospective, observational, 3-year follow-up study of a cohort of patients with stage 3 chronic kidney disease (CKD) from different nephrology departments of hospitals in the Spanish healthcare system. The primary study objective was to analyse risk factors for CKD progression, identifying possible differences between patients with and without diabetes mellitus (DM). The secondary objective was to analyse if the cardiovascular risk factors were also associated with CKD progression.
Patients and methodsA total of 462 patients (342 men and 120 women; mean age 66.5 ± 11.5 years) were recruited from 25 participating sites in Spain. Clinical, epidemilogical and analytical data were recorder in an electronic registrer each six months. Biological samples were obtained and frozen for a biobank record at baseline and at 18 and 36 months.
ResultsThe initial mean glomerular filtration rate estimated by MDRD and after that reestimated by CKD-EPI was 43.9 ± 7.9 mL/min/1.73 m2; and 29 ± 6,8 mL/min/1,73 m2 at 3 years. 27.3% of patients had microalbuminuria and 22.5% had macroalbuminuria. Two-thirds of the patients (66.2%) presented renal damage progression according to the study criteria (decrease of more than 15% in eGFR over the baseline value). 38.7% presented a reduction in eGFR ≥ 30%; 20.3% had a reduction in eGFR ≥40%; 10.4% had a reduction ≥50% and 6.9% had a reduction ≥57%. Of the 199 diabetics, 134 (67.3%) suffered renal damage progression. Of the 263 non-diabetics, 172 (65.3%) presented progression (p = 0.456). 27.3% of patients had microalbuminuria and 22.5% proteinuria. The study found that CKD progression to a higher stage was not greater in diabetic compared to non-diabetic patients. Multivariate analysis revealed that the presence of arterial hypertension bordered on significance as a progression factor in non-diabetic patients (p = 0.07), and that, in diabetic patients, lower calcium levels and elevated intact parathyroid hormone levels at baseline were associated with progression.
Conclusionin our study we have not found new factors for progression of renal damage, different from the yet well known traditional factors. DM “per se” was not a differential factor for progression in relation with non DM patients. Progression of renal damage in patients with CKD—3 KDOQI may be interpreted in a multifactorial context. The search for new biomarkers, different from traditional ones, is necessary to establish new therapeutic strategies to prevent the progression of CKD.
PROGRESER es un studio multicéntrio, prospectivo, observacional, con tres años de seguimiento, de una cohorte de pacientes con enfermedad renal crónica (ERC)-3 KDOQI, incluidos en servicios de Nefrología del Sistema Nacional de Salud en 14 Comunidades Autónomas (CCAA) de España. El objetivo primario fue analizar los factores de riesgo asociados con la progresión de la ERC, para identificar posibles diferencias entre pacientes con y sin Diabetes Mellitus (DM). El objetivo secundario fue investigar si los factores de riesgo cardiovascular (CV) están asociados con dicha progresión.
Material y métodosSe incluyeron 462 pacientes, (342 hombres y 120 mujeres, con edad media de 66.5 ± 11.5 años), reclutados en 25 centros. Se recogieron datos epidemiológicos, clínicos y analíticos cada seis meses, registrados en cuaderno electrónico. Se recogieron y congelaron muestras biológicas para biobanco basales y a 18 y 36 meses.
ResultadosEl filtrado glomerular estimado (FGe), calculado inicialmente mediante la ecuación Modification of Diet in Renal Disease (MDRD) y después recalculado mediante CKD-EPI, fue 43.9 ± 7.9 mL/min/1.73 m2 en el momento basal y 29,9 ± 6,8 mL/min/1,73 m2 a los tres años de seguimiento.
Dos tercios de los pacientes (66.2 %) presentaron progresión del daño renal según criterio del estudio (descenso mayor del 15 % del FGe sobre el valor basal). Un 38.7 % presentaron una reducción del FGe ≥ 30 %; un 20.3 % tuvieron una reducción del FGe ≥40%; un 10.4 % tuvieron una reduccion ≥50% y un 6.9 % una reducción ≥57%. De los 199 diabéticos, 134 (67,3 %) presentaron progresión. De los 263 no diabéticos, 172 (65,3%) presentaron progresión (p = 0,456). El 27.3% de pacientes presentaban microalbuminuria y el 22.5% proteinuria. El estudio mostró que la progresión de un estadio a otros más avanzados no fue superior en los pacientes con DM respecto a los no diabéticos. El análisis multivariante reveló que la presencia de Hipertensión Arterial (HTA) se aproximó a la significación estadística (p = 0.07) asociado a la progresión en los pacientes sin DM y que en los pacientes con DM unos niveles basales de calcio más bajos y de PTH-i más elevados sobre el valor basal tuvieron significación estadística como factores de progresión de la ERC.
ConclusiónNuestro estudio no ha revelado nuevos factores de progresión de daño renal con relación a los factores clásicos ya conocidos. No hemos encontrado diferencias significativas en cuanto a la progresión en pacientes con y sin DM La progresión del daño renal en pacientes con ERC-3 KDOQI debe interpretarse en un contexto multifactorial. Se precisa la búsqueda de nuevos biomarcadores, diferentes de los tradicionales, para establecer nuevas estrategias terapéuticas para prevenir la progresión de la ERC.
Early studies showed that chronic kidney disease (CKD) has an approximate prevalence of 9.1%.1 In Spain, the study EPIRCE (Epidemiology of CKD in Spain) found a prevalence of CKD-3 of’ 9%, which increased to 21.4% in people over 65 years of age.2 The EROCAP study estimated the incidence of CKD-3 in primary care clinics in Spain is 21.3%.3 Subsequently, data from the ENRICA study (Study of nutrition and cardiovascular risk in Spain) reported a national prevalence of 15.1%.4 This increase in the prevalence of CKD could be partially explained by the high rates of arterial hypertension (HTN) and diabetes mellitus (DM).5
Despite the improvement in our understanding of the pathophysiological mechanisms and therapeutic strategies of CKD, a high percentage of patients will experience progression of CKD to more advanced stages that will require renal replacement therapy (RRT).
Although the rate of progression is influenced by non-modifiable risk factors, such as race and age, there are other factors that could be modifiable, such as DM, obesity, hypertension, smoking and dyslipidemia, which may have an influence on such progression.3,6–8 In this regard, improving our knowledge of all these factors may be crucial from the pathophysiological and therapeutic objective point of view.
In recent years, numerous studies have revealed the existence of new factors that may play a significant role in the progression of CKD.8–11 However, there are no studies designed with specific criteria to establish the role of these new progression factors in an integrated manner that includes clinical, biochemical and genetic factors.
CKD is not only associated with the inexorable deterioration of renal function, but also entails an increased risk of cardiovascular (CV) disease and mortality compared to the general population.12 Furthermore, the possibility of death from CV factors is greater than that of starting RRT.7 Although these facts are well-known, the increase in morbidity and mortality associated with CKD has not been well clarified, especially in the early stages of CKD.2,13–19 In the present study we have sought to examine which factors may be associated with the progression of renal and CV damage in patients with CKD stage 3, defined according to the criteria of the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, analyzing possible differences between patients with and without DM.
MethodsStudy designThe PROGRESER study (PROGRESion Factors in Chronic Kidney Disease) is an observational, prospective, multicenter study in a cohort of patients with KDOQI CKD stage 3, conducted in Nephrology departments from various hospitals in Spain, with an initial follow-up of three years. The study was approved by the Spanish Agency of Medications (AEMPS) on September 29, 2010 as a post-authorization observational study. The study protocol was approved by the Clinical Research Ethics Committee of the Hospital Universitari de Bellvitge, Hospitalet de Llobregat, Barcelona, as well as by the ethics committees of the various participating centers (see Appendix AAppendix 1). The primary objective of the study was to assess the risk factors associated with the progression of kidney damage in patients with KDOQI CKD stage 3. The secondary objectives were to describe the hospitalization and mortality data and the factors related.
Inclusion and exclusion criteriaInclusion criteria were: age equal to or greater than 18 years; diagnosis of CKD stage-3 according to the KDIGO guidelines, defined as an estimated glomerular filtration rate (eGFR) between 60 and 30 mL/min/1.73 m2 in two successive determinations separated by at least 3 months of interval, (using the Modification of Diet in Renal Disease (MDRD)-420 equation), thereafter in the global analysis, the eGFR was re-estimated using the Epidemiology of Chronic Kidney Disease (CKD-EPI) equation, that may or may not be accompanied by albuminuria (expressed as the albumin/creatinine ratio in urine) equal to or greater than 30 mg/g in the first morning urine sample collected. A life expectancy greater than one year and signed informed consent.
Exclusion criteria wereAge less than 18 years, CKD not in stage 3. Neoplasia disease, active infection; proteinuria greater than 3 g/day; psychiatric illness or inability to sign informed consent; pregnancy or breastfeeding.
Patients were successively included in the outpatient clinics of the Nephrology services of the participating centers, between January 2012 and September 2015. All patients signed consent forms, in accordance with the Declaration of Helsinki.
Data collectionAll patients had a baseline visit where informed consent was signed, electrocardiogram (ECG), sociodemographic questionnaire and samples were obtained (blood, urine, plasma, serum and rests of cells).
Hematological and biochemical determinations were performed every six months. ECG was obtained at baseline and at 12, 18 and 36 months. Data related to treatments were collected, and also as well as events with hospitalization that occurred during the study follow-up.
A specific consent form was used to allow for the subsequent analysis of the biobank samples. Blood, plasma, urine and cellular remains were collected at baseline, 18 and 36 months and frozen at −80 °C at each center until they were sent to the REDinREN biobank, Renal Research Network, at the University of Alcalá de Henares, Madrid. The results of the genetic polymorphisms will be the subject of a subsequent study.
The sample size was estimated from the data regarding the eGFR obtained in the MERENA21 study. The initial “n” was estimated in 630 patients, considering a type I error of 5% and a type II error of 5%, to obtain a significance level of p < 0.05. Progression of CKD was considered to have occurred when the eGFR decreased by 15% or more from the baseline value or when the patient transitioned to a more advanced stage of CKD (CKD stage 4, provided that progression was greater than a 15% decreae in GFR). The analysis of results was subsequently stratified considering whether the percentage reduction in eGFR was greater than 30%, 40% or 50% from the baseline value.
All data were recorded every six months on a website (https://www.e-clinical.org/progreser) from baseline (month 0) until the end of the three-year follow-up. The variables included in the study are shown in Supplementary Appendix Table S1. To assess the existence of left ventricular hypertrophy (LVH), the Cornell voltage-duration product was calculated (see Supplementary Appendix Table S1). The definition of the variables HTA, DM, obesity and overweight, and heart failure is shown in the footnote of Supplementary Appendix Table S1.
Statistical analysisQuantitative variables are described using central tendency and dispersion measurements (mean and standard deviation [SD] or mean and interquartile range [IQR] depending on the normal or nonparametric distribution of the variable). The number of patients to complete the total “n” includes patients with missing data. Qualitative variables are described using absolute (n) and relative (%) frequencies. For the comparison of qualitative variables, the Chi-square test or Fisher's exact test were used, depending on the percentage of data obtained with an expected frequency of 5 or less. The Student t test or the Mann-Whitney U test were used to compare two means, depending on the normality of the data distribution. For the comparison of the eGFR between patients with and without DM, the General Linear Model for Repeated Measures was used. Statistical significance was calculated by providing the statistical significance for each group regarding the evolution (Hotteling's trace) and using Fisher's multiple comparison test (LSD method [Least significant difference]).
To construct the binary logistic regression, the dependent variable (progression/non-progression) and the independent variables were defined. To identify possible factors influencing the response, a bivariate analysis was first performed, and variables with statistical significance or close to it (p < 0.20) were considered as possible factors to be included in the model. Once the variables were identified, the regression model was built by forward stepwise regression or by automatic variable selection. Statistical significance was considered for values of p < 0.05. The data were analyzed using SPSS v.18 or higher. For predictive variables associated with the primary and secondary objectives, the 95% confidence interval (CI) was estimated when necessary.
ResultsGeneral characteristics of the study patients (Table 1)Initially, there were 487 patients included, recruited in 25 different centers, however 25 patients were discarded because they did not meet inclusion criteria. Thus, the final number of patients analyzed was 462 (342 men and 120 women, with a mean age of 66.5 ± 11.5 years). Their clinical and anthropometric characteristics, as well as the causes of kidney disease and the CV risk factors that they have, are presented in Table 1. Baseline laboratory data are exposed in Table 2.
General characteristics of the patients included.
| Variables | n | Percentage, mean ± standard deviation or median (interquartile range) |
|---|---|---|
| Age, years (mean) | 462 | 65.5 ± 11.5 |
| Sex | 462 | |
| Male | 342 (74%) | |
| Female | 120(26%) | |
| Location | 462 | |
| Rural | 106 (11.9%) | |
| Urban | 353 (76.4%) | |
| Level of education | 459 | |
| No studies | 68 (14.8%) | |
| Primary school | 337 (73.4%) | |
| Higher education | 54 (11.8%) | |
| Diabetes mellitus | 462 | |
| No DM | 263 (56.9%) | |
| DM1 | 13 (2.8%) | |
| DM2 | 186 (40.3%) | |
| Hypertension (HTN) | 462 | 441 (95.5%) |
| Heart failure with hospitalization | 455 | 49 (10.8%) |
| Ischemic heart disease | 456 | 84 (18.4%) |
| Cerebrovascular disease | 457 | 55 (12%) |
| Peripheral vascular disease | 456 | 79 (17.3%) |
| Smoking | 462 | |
| Active smoker | 70 (15.2%) | |
| Ex-smoker | 226 (49.1%) | |
| Never smoker | 164 (35.7%) | |
| Etiology of kidney disease | 461 | |
| Glomerular | 30 (6.5%) | |
| Interstitial 35 | 35 (7.6%) | |
| Vascular/nephroangiosclerosis | 168 (36.4%) | |
| Polycystic disease/cystic disease | 31 (6.7%) | |
| Diabetic kidney disease | 199 (42.6%) | |
| Unidentified/other | 39 (8.5%) | |
| Left ventricular hypertrophy (Cornell voltage-duration product > 2440) | 411 | 79 (19.2%) |
| Height (cm) | 450 | 165.5 ± 10.0 |
| Weight (kg) | 451 | 80.4 ± 14.6 |
| BMI, kg/m2 | 446 | 29.44.9 |
| Waist circumference (cm) | 404 | 101.5 ± 13.0 |
| Heart rate, bpm | 455 | 72.9 ± 12.8 |
| Systolic blood pressure, mmHg | 461 | 138.7 ± 17.5 |
| Diastolic blood pressure, mmHg | 461 | 76.5±10.9 |
| Pulse pressure | 459 | 62.2 ±16.2 |
Laboratory data at baseline.
| Variables | n | Mean ± standard deviation or median [interquartile range] |
|---|---|---|
| Glucose (mg/dL) | 460 | 118 ± 42 |
| HbA1c (in DM) (%) | 277 | 6.9 ± 1.3 |
| Urea (mg/dL) | 456 | 67.2 ± 20.5 |
| Creatinine (mg/dL) | 462 | 1.6 [1.4–1.8]a |
| Estimated glomerular filtration rate (eGFR) by CKD-EPI, mL/min/1.73 m2 | 462 | 43.9 ± 7.9 |
| Uric acid (mg/dL) | 456 | 6.8 ± 1.5 |
| Total cholesterol (mg/dL) | 460 | 176.7 ± 40.3 |
| Triglycerides (mg/dL) | 445 | 149.6 ± 91.5 |
| LDL cholesterol (mg/dL) | 386 | 101.9 ± 33.0 |
| HDL cholesterol (mg/dL) | 394 | 47.4 ± 13.7 |
| Non-HDL cholesterol (mg/dL) | 393 | 131.8 ± 37.4 |
| AST/GOT (mU/mL) | 398 | 22.3 ± 8.7 |
| ALT/ GPT (mU/mL) | 445 | 19.0 [15.0−26.2]a |
| GGPT(mU/mL) | 421 | 25.0 [16.0−37.0]a |
| Calcium (mg/dL) | 454 | 9.5 [9.7−9.8]a |
| Phosphorus (mg/dL) | 450 | 3.4 (3.0−3.8)a |
| intact PTH (pg/mL) | 367 | 69.5 [48.0−100.0]a |
| 25-OH vitamin D (ng/mL) | 270 | 24.4 ± 12.5 |
| Iron (mg/dL) | 341 | 77.4 ± 24.0 |
| Ferritin (ng/mL) | 418 | 102.0 [50.8−182.4]a |
| Transferrin saturation index (%) | 327 | 24.0 [19.0−30.0]a |
| Hematocrit (%) | 458 | 41.0 ± 4.9 |
| Hemoglobin (g/dL) | 459 | 13.7 ± 1.7 |
| Platelets (/mm3) | 458 | 212802 ± 66189 |
| Leukocytes (/mm3) | 458 | 7250.0 [6100.0−8577.5]a |
| Neutrophils (%) | 455 | 59.9 ± 8.8 |
| Lymphocytes (%) | 456 | 27.5 ± 8.0 |
| Urine albumin/creatinine ratio (mg/g) | 396 | 47.7 [10.0−309.6]a |
The study included 199 patients whose cause of CKD was attributed to DM by the participating investigators. In these patients with DM, 18.6% were receiving insulin, 13% metformin, 10.6% DPP-4 inhibitors, and 22.1% other hypoglycemic agents, alone or in combination. No patient was receiving treatment with renal sodium-glucose transporter type 2 inhibitors (SLGT2i) and only one patient was receiving treatment with glucagon-like peptide type 1 receptor agonists (GLP-1RAs), drugs that would be introduced into routine clinical practice for the management of DM years after the start of our study (Appendix A Supplementary Table S1).
Regarding previous CV risk factors, 18.4% of patients had a history of coronary artery disease, 12% stroke, 17.3% peripheral vascular disease, and 10.8% heart failure.
At baseline, 41.9% of patients had normal albuminuria, 31.8% had microalbuminuria, and 26.3% had proteinuria. Tables 1 and 2 show the remaining clinical, anthropometric, and laboratory characteristics.
Progression of renal damageTwo-thirds of patients (66.2%) (95% CI: 61.7–70.5) presented deterioration of renal function during the follow-up period, defined as a decrease of more than 15% in eGFR as compared with the baseline value; 38.7% had a reduction in eGFR equal to or greater than 30%; 20.3% had a reduction in eGFR ≥40%; 10.4% had a reduction ≥50%, and 6.9% had a reduction ≥57%. Out of the 199 patients with DM, 134 (67.3%) presented progression of renal damage. Of the 263 non-diabetics, 172 (65.3%) showed such a progression (p = 0.456).
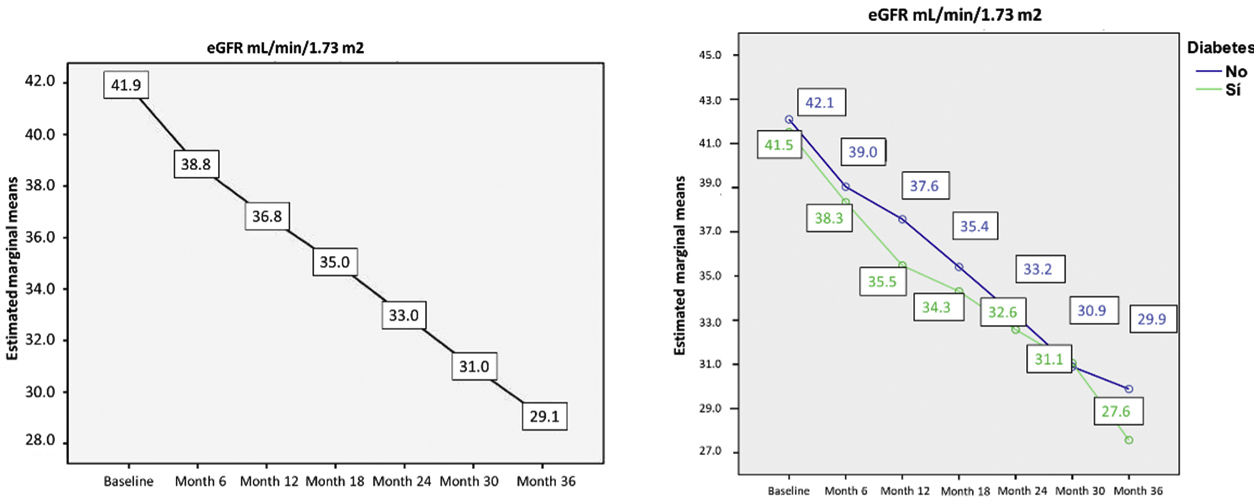
Table 3 shows the differences in eGFR between visits compared to baseline, showing a progression of renal damage of 4.5 mL/min/1.73 m2 during the first year, 4.3 mL/min/1.73m2 in the second year, and 3.4 mL/min/1.73 m2 in the third year.
Differences in estimated glomerular filtration rate between visits compared to baseline.
| Month | Difference between means | Standard error | p | 95% CI difference between means Upper limit Lower limit | |
|---|---|---|---|---|---|
| 6 | 3.051 | 0.439 | 0.000 | 2.185 | 3.917 |
| 12 | 4.522 | 0.468 | 0.000 | 3.597 | 5.447 |
| 18 | 6.683 | 0.540 | 0.000 | 5.617 | 7.750 |
| 24 | 8.887 | 0.527 | 0.000 | 7.847 | 9.926 |
| 30 | 11.199 | 0.560 | 0.000 | 10.094 | 12.304 |
| 36 | 12,209 | 0.624 | 0.000 | 10.978 | 13.439 |
p value calculated using Fisher's LSD (least significant difference) multiple comparison test.
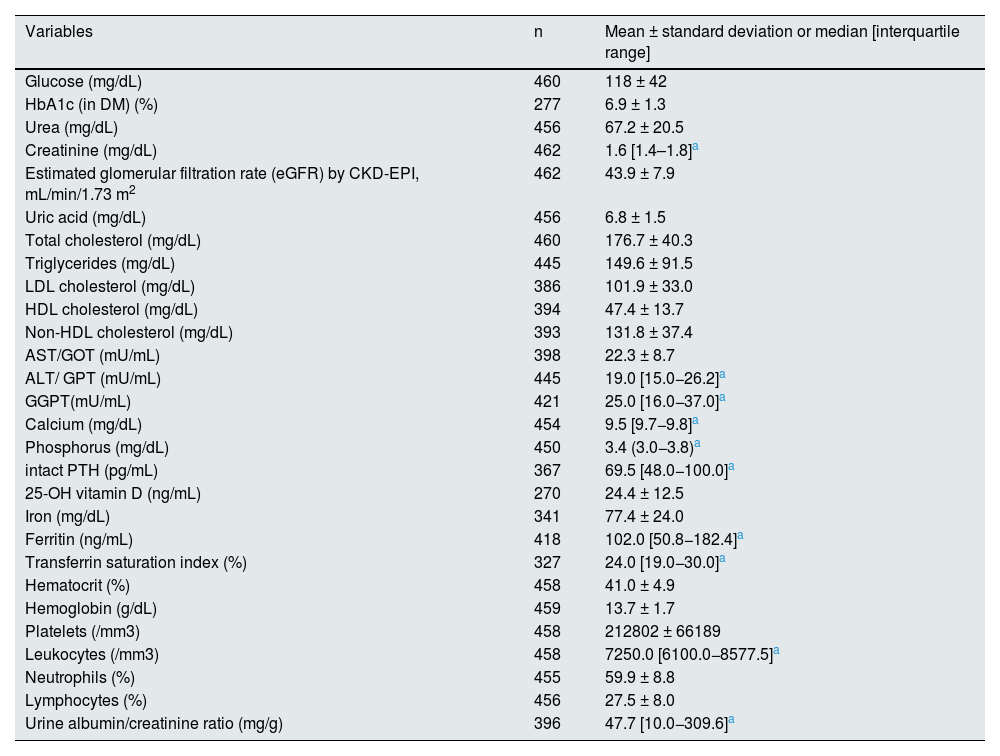
Table 4 shows the differences in eGFR between patients with and without DM throughout the study. No significant differences in eGFR were detected when comparing patients with and without DM at all study visits.
Differences in the mean of estimated glomerular filtration rate between patients with and without diabetes mellitus during study visits.
| Visits | Diabetics 179 (38.7%) | Non-diabetics 283 (61.3%) |
|---|---|---|
| Baseline | 41.5 | 42.1 |
| 6 months | 38.3 | 39.0 |
| 12 months | 35.5 | 37.6 |
| 18 months | 34.3 | 35.4 |
| 24 months | 32.6 | 33.2 |
| 30 months | 31.1 | 30.9 |
| 36 months | 27.6 | 29.9 |
p = n.s. in all study visits.
At the end of the study at 36 months, the mean eGFR decreased by 36%, from 41.9 ± 7.9 mL/min/1.73 m2 to 29.1 ± 9.6 mL/min/1.73 m2 (p < 0.001) (Fig., left). There was no statistical difference in the decrease in eGFR, estimated by CKD-EPI, between patients with DM without DM (p = 0.325) (Fig. 1, right).
Evolution of estimated glomerular filtration rate during the study. Left: evolution in estimated glomerular filtration rate in all patients in the study. Right: evolution in estimated glomerular filtration rate separated by groups (diabetic and non-diabetic). No statistically significant differences were observed in the evolution of CKD-EPI in non-diabetic as compared with diabetic patients (p = 0.325, p value was calculated in the intersubject effects test).
In the bivariate analysis, the factors associated with progression of renal damage were a history of cerebrovascular disease, lower triglyceride levels, and lower Ca levels (p = 0.045, p = 0.004, and p = 0.042, respectively). In the multivariate analysis, the lowest calcium level remained as an independently associated factor with the progression of renal damage (OR: 0.548; 95% CI: 0.353–852; p = 0.007). In patients with DM, the independent factors of worse prognosis were active smoking (OR: 6.620; 95% CI: 1.718–25.513; p = 0.006) and higher levels of PTH-i (OR: 1.014; 95% CI: 1.004–1.023; p = 0.003).
The bivariate analysis of risk factors for progression of kidney damage (progression vs. non-progression) is shown in Supplementary Appendix Table S3. Regarding the continuous variables, it was observed that patients who presented progression of renal damage showed significantly lower serum calcium values than those in whom such damage did not progress (median: 9.5; IQR [9.2–9.8] vs 9.6 [9.3–9.9], p = 0.004).
Table 5 shows the risk factors associated with the progression of renal damage, considering as progression not only the criterion of the study but also a decrease in eGFR of 30%, 40% or 50% with respect to baseline eGFR. In all cases (progression of 30%, 40% and 50%). The factors with independent prognostic weight that were associated with the presence of progression of renal damage in the three percentages of reduction of eGFR were the presence of microalbuminuria, proteinuria and the levels of calcium and PTH-i.
Progression factors for chronic kidney disease according to the percent reduction in estimated glomerular filtration rate (eGFR) considered as dependent variable (reduction of >30%, >40% or >50%).
| Reduction in eGFR > 30% | p | Odds ratio (95% CI) |
|---|---|---|
| Cerebrovascular disease (0 = No, 1 = Yes) | 0.017 | 0.372 (0.165−0.839) |
| LDL cholesterol (mg/dL) (0 = <100 mg/dL, 1 = ≥100 mg/dL) | 0.046 | 1.589 (1.008−2.504) |
| Baseline urinary albumin/creatinine ratio (mg/g); reference: normal <30 mg/g | 0.024 | |
| Microalbuminuria 30−299 mg/g | 0.060 | 1.673 (0.979−2.856) |
| Proteinuria ≥300 mg/g | 0.009 | 2.134 (1.211−3.760) |
| Calcium (mg/dL) | 0.030 | 0.566 (0.339−0.947) |
| Reduction in eGFR >40% | ||
| Basal urine albumin/creatinine ratio (mg/g); reference: normal <30 mg/g | 0.001 | |
| Microalbuminuria 30−299 mg/g | 0.130 | 1.988 (0.817−4.839) |
| Proteinuria ≥300 mg/g | 0.000 | 5.980 (2.592−13.793) |
| Calcium (mg/dL) | 0.006 | 0.344 (0.160−0.741 |
| Intact PTH (pg/mL) | 0.013 | 1.008 (1.002−1.015 |
| Reduction in eGFR>50% | ||
| Basal urine albumin/creatinine ratio (mg/g); Reference: normal <30 mg/g | 0.011 | |
| Microalbuminuria 30−299 mg/g | 0.150 | 2.355 (0.734−7.556) |
| Proteinuria ≥300 mg/g | 0.003 | 5.362 (1.744−16.482) |
| Calcium (mg/dL) | 0.032 | 360 (0.141−0.916) |
| Intact PTH (pg/mL) | 0.013 | 1.010 (1.002−1.018) |
eGFR: estimated glomerular filtration rate by CKD-EPI; CI: confidence interval.
Cerebrovascular disease: includes transient ischemic attack and stroke.
The data are the result of the analysis of renal deterioration through a descriptive analysis of cases with a reduction greater than or equal to 30%, 40% or 50% in the last recorded value of eGFR using CKD-EPI, compared to the baseline value.
No significant associations were detected between renal progression and the presence of diabetes mellitus, BMI, hypertension, history of heart failure, Cornell index in the ECG, baseline glomerular filtration rate, triglycerides, HDL cholesterol, iron, ferritin, TSI and phosphorus.
During the follow-up period, 20.6% of patients required hospitalization, with no differences in patients with and without DM (23.6% of patients with DM and 18.3% of patients without DM (p = 0.172). A1.7% of the patients started dialysis. Overall mortality throughout follow-up was 6.1% (43% from CV causes, 21% from tumors, 14% from infections, and 22% from other causes); mortality was significantly associated with a history of coronary artery disease (p < 0.001; 95% CI: 2.111–10.386). Follow-up was lost in 1 out of 10 patients (10.4%).
Risk factors associated with hospitalization and mortalityIn the bivariate regression analysis, the results showed that the variables predictive of a higher risk of hospitalization were: the presence of DM (OR: 1.370; 95% CI: 0.871–2.153), HTN (OR: 5.418; 95% CI: 0.718–40.893), a Cornell index greater than 28 mm in men and 20 mm in women (OR: 1.547; 95% CI: 0.833–2.874), a Cornell voltage greater than 2,440 mm × ms (OR: 1.902; 95% CI: 1.101–3.283), a hemoglobin concentration <11 g/dL (OR: 2.736; 95% CI: 1.085–6.902), an HbA1c level ≥7% (OR: 1.508; 95% CI: 0.829–2.742) and a fasting blood glucose ≥126 mg/dL (OR: 1.372; 95% CI: 0.849–2.218).
Of the parameters analyzed as continuous variables, higher calcium levels were associated with a lower risk of hospitalization (OR: 0.988; 95% CI: 0.977–1.000), as it was the transferrin saturation index (TSI) (OR: 0.973; 95% CI: 0.946–1.002).
Variables associated with increased mortality were active smoking (p < 0.2) (OR: 2.698; 95% CI: 1.064–6.842), history of heart failure requiring hospitalization (OR: 2.558; 95% CI: 0.979–6.684), coronary artery disease (OR: 4.682; 95% CI: 2.111–10.386), Cornell voltage >2,440 mm × ms (OR: 2.243; 95% CI: 0.967–5.201), and HbA1c >7% (OR: 2.353; 95% CI: 0.813–6.808). Low levels of Ca (OR: 2.292; 95% CI: 1.006–5.225) and high levels of creatinine (OR: 3.027; 95% CI: 0.860–10.66) were associated with higher mortality in the bivariate analysis.
In the final logistic regression model to predict mortality, the independent predictive factors were the presence of coronary artery disease (OR: 4.628; 95% CI: 2.11–10.28), with higher levels of Ca being protective against hospitalization (OR: 0.460; 95% CI: 0.273−0.774).
DiscussionThe primary objective of the study was to assess the risk factors associated with the progression of renal damage in patients with CKD-3 KDOQI, with special interest in verifying whether there were differences between the population with and without DM. The secondary objectives were to describe the hospitalization and mortality data and the related factors.
In our study, the factors that favor the progression of renal damage in CKD patients were related to parameters of bone and mineral metabolism in patients with DM (lower baseline calcium levels and higher iPTH), and probably with the presence of hypertension in patients without DM (p level close to statistical significance: p = 0.07). Traditional risk factors, such as diabetes, obesity, smoking and dyslipidemia, were not significantly associated with the progression of renal damage in the multivariate analysis. Although smoking (both current and past smoking), a history of previous cerebrovascular disease, and hypertriglyceridemia were associated with the progression of renal damage in the DM population in the bivariate analysis, they lost their significant association with the progression of renal damage as independent variables in the multiple regression analysis.
Some recent studies have attempted to elucidate which factors have the greatest impact on the development and progression of renal damage. For example, in our MERENA study,22 data on 1,156 patients with CKD stage 3 and 4 showed that several of the CV risk factors (HTN, coronary artery disease, cerebrovascular disease, heart failure, or peripheral vascular disease) are very frequently present and are more prevalent in the CKD population, regardless of the presence of DM. When we analyzed mortality after five years of follow-up in this study, it was found that hospitalizations were more frequent in the group of patients with DM (44% due to CV causes) and that in 44% of patients the CKD progressed to CKD stage 5, requiring RRT (35.9% by hemodialysis and 8.1% by peritoneal dialysis), but mortality was not significantly higher in the group of patients with DM compared to patients without DM (17.2% vs. 15.4%; p = 0.17) (data presented at the S.E.N. Congress in Seville, October 15–18, 2011).23 Similar data have been described in the PECERA study, which included 995 patients with CKD-KDOQI 4, 35% of them with DM, and with a follow-up of 3 years. In this study, the main cause of hospitalizations was CV diease, the most important being heart failure. Of these patients, 32% required RRT (27% by hemodialysis, 4% by peritoneal dialysis and 1% received a kidney transplant). During the follow-up, 15% of them died (46.2% due to CV causes).24
We believe it is important to point out that the percentage of progression in these two previously mentioned studies22,24 and in the one being reported now, although high, is not that excessively high when compared with older data from the literature,. This could be due in part to the insistence of guidelines and consensus documents on a greater control of progression factors such as hypertension, dyslipidemia, anemia or bone and mineral metabolism parameters. In this context, it is also important to note that the patients in our study did not benefit from the new antihyperglycemic drugs with potential nephroprotective and cardioprotective effects, such as SGLT2 or GLP-1 inhibitors, which were not yet introduced for clinical use in the those years when our patients were included in the study.
In the NEFRONA study, which included 4,137 patients with CKD stages 3–5 without previous CV history and 843 control subjects without known kidney disease, the factors associated with worse CV prognosis were the progression of carotid atheroma plaque, smoking, the presence of DM or systolic hypertension, low levels of 25-OH vitamin D, as well as the absence of treatment with phosphorus binders.25 A subsequent subanalysis of the same study in a cohort of 1,152 patients showed that the factors for progression of kidney damage were higher levels of P in CKD- 3 to 5, decreased levels of 25-OH vitamin D, and i-PTH levels higher than 110 pg/mL in CKD 4 and 5.26
In the RIACE (Renal Insufficiency and CV Events) study, a multicenter, observational study that included 15,733 patients with type 2 DM, the most striking data were the high number of patients without albuminuria (the predominant phenotype), who also showed a high prevalence of CV risk factors, the poor concordance between the stage of CKD and the presence of diabetic retinopathy, as well as the administration of oral hypoglycemic agents not recommended with eGFR <30 mL/min/1.72 m2.27
A meta-analysis published in 2016 evaluated twelve studies of CKD progression with more than 13,000 patients and showed an average progression to CKD-5 of 40 events/1,000 patients/year, a mortality of 41 episodes/1,000 patients/year, with CV episodes of 29/1,000 patients/year. It should be noted that the authors observed a great variability of results between different countries.28
We find it interesting to highlight that the estimation of GFR using equations that include plasma creatinine may show variability in the the classification y the stage of CKD in these patients. When we began the present study, the equation available in the laboratories of the different centers was the MDRD in its different formulas, with 4, 6 and MDRD-IDMS as the most used, the latter being the one selected to classify the patients included. New equations have been subsequently introduced, the most used being the CKD-EPI. We therefore had to recalculate the GFR both at baseline and throughout the study and at three years of follow-up.
As a result of this re-estimation, 19 patients were reclassified as CKD-4. No other changes were detected in the reclassification of CKD stage. This data may be important when estimating the degree of alteration in renal function and the possible consequences of the use of medications which dose is modified according to the GFR, to avoid possible nephrotoxicity or other adverse effects. However, the analysis of patients with progression renal damage, as well as the risk factors associated with it, was carried out in the multivariate analysis, and no differences were detected with the use of the MDRD and the CKD-EPI formula.
We must recognize several limitations in our study. The first is that we did not manage to include the number of patients initially proposed due to difficulties in recruitment, so the closing period had to be extended significantly. Second, the fact that we did not reach the proposed “n” value could have influenced the failure to demonstrate statistical significance in the multivariate analysis of some of the progression factors proposed in the bivariate analysis. This same fact could explain the lack of statistical significance of albuminuria and proteinuria in the multivariate analysis, a fact aggravated by the loss of these two results in some patients.
Although the study has these limitations, especially the lack of detection of risk factors in the clinical variables, this observation is not infrequent, especially in patients from large studies, since they usually have adequate control of risk factors (hypertension, dyslipidemia, etc.) and the event rate is usually low. A recent example is the EMPA-KIDNEY study,29 which analyzed the effect of empagliflozin in 6,609 patients with CKD and risk of progression of kidney damage, including diabetics and non-diabetics, where 65.8% had CKD stage 3 and 34.2% had CKD stage 4. The mean eGFR was 37.4 ± 14.4 mL/min/1.73 m2 (in our study the mean eGFR was 43.9 ± 7.9 mL/min/1.73 m2 and the percentage of normo, micro and macroalbuminuria was 52%, 28% and 20%, very similar to the data from our study: 41.9%, 31.8% and 26.3%). The rate of events related to the combined primary endpoint (reduction of eGFR ≥40%, reaching an eGFR of 10 mL/min/1.73 m2 or starting dialysis) was only 18% in patients with proteinuria and 6% and 7% in patients with normo- and microalbuminuria. This may be a common aspect with our study. That is why the search for new biomarkers in the samples stored in the biobank may become important in the near future.
Despite these limitations, we believe that the strengths of our study lie in the fact that it included a broad representation of patients all over Spain (from14 of the 17 autonomous communities), the follow-up period was relatively long (three years), and the sample included a significant number of patients with DM. The fact that that patients did not receive treatment with SLGT2 inhibitors or GLP-1 RAs, may be used to compare the evolution of our patients as a control group for future studies that evaluate the progression of kidney damage and CV damage with that of other patients treated with these drugs, versus those treated as classically with renin-angiotensin-aldosterone system blockers, in addition to standard treatments. An important additional strength is the availability of biological samples in the biobank, particularly urine and cell, which may allow us to evaluate new biomarkers that may help to identify factors related with progression of kidney damage and establish new therapeutic targets.
A good example of the benefit of saving biological samples for future analysis is the study recently published in Nephrology, Dialysis and Transplantation, in which patients from the PROGRESER cohort were analyzed together with patients from the PRONEDI study.30 The authors identified the urinary protein Dickkopf-3 as a new marker of progression and death in patients with CKD stages 2 and 3.31 This study highlights the importance of having biological samples in translational research that assess not only aspects related to traceability, but also the connection with clinical characteristics of the patients, which is fundamental for future research.32
We believe that the progression of kidney damage must be interpreted in a multifactorial context. It is necessary to search for new biomarkers, different from the traditional ones, in order to establish therapeutic strategies able to prevent the progression of CKD.
ConclusionsOur study has not revealed new factors of progression of kidney damage in relation to the factors already known. The study has shown in the multivariate regression analysis that factors such as hypertension may be related to the progression of kidney damage in the population with non-diabetic CKD, and that low calcium levels and high intact PTH levels were correlated with progression of kidney damage in patients with DM and CKD-stage 3 KDOQI. We have not found significant differences in terms of the progression of CKD in patients with and without DM, nor in the factors related to such progression.
FundingThe project received funding from Laboratorio Esteve, Barcelona, and the SENEFRO Foundation.
Our thanks to the members of GEENDIAB and to all researchers who have made possible the PROGRESER study. To Laboratorio ESTEVE that has financed the entire study. To CRO DYNASOLUTIONS for its technical assistance. To Antonio Torres Collado and MSG Consulting for their editorial assistance. AMC, JLG, AO and JFNG are members of the REDinREN of the Instituto de Salud Carlos III and of RICORS 2040: RD12/0021/0019, RD16/0009/0022 and RD 21/0005/0013.