Patients with chronic kidney disease (CKD) are at high risk of cardiovascular morbidity and mortality. Subclinical cardiac structural alterations have prognostic value in these patients. The aim was to analyse the prevalence of valvular calcification, the evolution and the relationship with different risk factors.
Material and methodsPart of the sample of the NEFRONA study was randomly selected. Aortic and mitral valve calcification were analysed in echocardiograms performed at the baseline visit and at 24 months.
ResultsWe included 397 patients, the estimated basal glomerular filtrate (eGFR) was 33 ml/min with significant decrease to 30.9 ml/min. There was an increase in the area of carotid and femoral plaque, as well as an increase in patients with aortic and mitral calcification at 24 months. A positive association of mitral calcification at 24 months with age, ankle-brachial index (ABI) and calcium-phosphorus product (CaxP) at baseline visit was observed, without association with eGFR. Aortic calcification at 24 months was positively associated with age, phosphorous and total carotid plaque area at baseline, with no relationship to eGFR.
ConclusionsA significant prevalence of valvular calcification was observed in patients with CKD without known cardiovascular disease.Two-year progression was observed independently of the eGFR. Patients with higher risk of mitral valve calcification were those with older age, higher ABI and CaxP product. Patients with a higher risk of aortic calcification were those with older age, higher phosphorous levels and larger area of carotid plaque. Identifying these higher risk patients would help to avoid future cardiovascular events intensifying follow-ups.
Los pacientes con enfermedad renal crónica (ERC) tiene alto riesgo de morbimortalidad cardiovascular. Las alteraciones estructurales cardiacas subclínicas tienen valor pronóstico en estos pacientes. El objetivo fue estudiar la calcificación valvular, su evolución y relación con diferentes factores de riesgo.
Material y métodosSe seleccionó aleatoriamente parte de la muestra del estudio NEFRONA analizando la calcificación valvular aórtica y mitral en ecocardiogramas de la visita basal y a los 24 meses.
ResultadosSe estudiaron 397 pacientes con filtrado glomerular estimado (FGE) basal de 33 ml/min/1.73 m2 con disminución significativa hasta 30.9 ml/min/1.73 m2 Se produjo aumento del área de placa carotidea y femoral, así como aumento de los pacientes con calcificación valvular a los 24 meses. Se observó asociación positiva de la calcificación mitral a 24 meses con la edad, el índice tobillo brazo (ITB) y el producto calcio fósforo (CaxP) basal, sin asociación con el FGE. La calcificación aórtica a los 24 meses presentó asociación positiva con el área total de placa carotidea, el fósforo y la edad basal, sin relación con el FGE.
ConclusionesSe objetivó en pacientes con ERC, sin enfermedad cardiovascular conocida progresión de la calcificación valvular a dos años independientemente del FGE. Presentaron mayor calcificación valvular mitral aquellos de mayor edad, mayor ITB y producto CaxP. Presentaron mayor calcificación valvular aórtica aquellos de mayor edad, mayores niveles de fósforo y mayor área total de placa carotidea. La identificación de estos pacientes con mayor riesgo, podría ayudar a evitar eventos cardiovasculares futuros intensificando el seguimiento.
During the last decades Chronic kidney disease (CKD) has become one of the main public health problems.1,2 The analysis of data worldwide has revealed that about 500 million adults have CKD.1,3 The epidemiological significance of CKD is based on two fundamental aspects, the renal replacement therapy, which affects only 1% of these patients but it represents a significant reduction in quality of life and is the most expensive treatment for chronic diseases and consume a 5% of health budgets1; a second important problem is that since the initial stages, CKD causes a very significant increase in the risk of cardiovascular morbidity and mortality and mortality of any cause.2,4,5
In 2018, it was published the Analysis of the Study of Nutrition and Cardiovascular Risk in Spain (ENRICA), a national epidemiological study, in a sample of 11,505 subjects representative of the Spanish adult population. The objective was to estimate the prevalence of CKD and evaluate the impact of cardiovascular risk factors (CVRF) on CKD. The main result was the observation that 1 out of 7 adults in Spain presented CKD (15% of the population). CKD was found more frequently in men, elderly subjects and with cardiovascular disease (CVD) or CVRF. The observation of a continuous and growing relationship between the prevalence of CKD and the accumulation of CVRF suggests that in the general population, CKD may be considered a cardiovascular condition6 and signifies a notable potential for prevention in this group of patients.
CVD is the leading cause of death in patients with CKD7 with an increase in cardiovascular risk of up to 20 times that of the general population, even in the early stages of CKD.8–10 Up to 80% of CKD patients present associated CVD: arterial hypertension (HTN) (36%), ischemic heart disease (22–39%), atrial fibrillation (30%), valvular heart disease (24%) and left ventricular hypertrophy (LVH) ) (50–75% in stages 3–4 of CKD).11
There are various models capable of stratifying the cardiovascular risk of patients which would makes possible to adapt the treatment and prevention measures. However, the existence of cardiovascular events has been observed in groups of patients with calculated low-moderate risk,12 therefore these models do not fit precisely to patients with CKD. This finding suggests the existence of specific CRFs in this profile of patients who are referred to as non-classical or emerging risk factors.13
Non-classical or emerging risk factors are many: alterations in bone mineral metabolism (levels of phosphorus (P), vitamin D, parathyroid hormone (PTH), the use of phosphate binders), lipid biomarkers (Lipoprotein a, Apolipoprotein A1 and B etc.), inflammatory biomarkers ( C reactive protein (CRP), interleukins 1, 6 and 18, tumor necrosis factor α), biomarkers of hemostasis (D-dimer, fibrinogen, homocysteine etc.), renal biomarkers (uric acid, ferritin, uric acid, microalbuminuria, FGF-23) among others that have been the object of research during the last years.14
Subclinical structural cardiac and vascular alterations, notably LVH, dilation of the left atrium (LA), carotid atheromatosis, peripheral vascular disease, and cardiac valve calcification, are frequently detected in this patient profile and have prognostic value, correlating with high cardiovascular morbidity and mortality.15–17 These alterations have been associated with numerous traditional or classic risk factors as age, hypertension, diabetes mellitus (DM) or dyslipidemia (DL), but also non-traditional or emerging factors listed previously.18 The detection by means of non-invasive or minimally invasive techniques of the described structural alterations, as well as of the risk factors, would allow the identification of patients with a higher cardiovascular risk, helping to optimize patient’s therapy and follow-up, thus reducing their morbidity and mortality.19,20
Cardiac imaging is very helpful, especially echocardiography, given its low cost, portability, and the absence of radiation or contrast as opposed to cardiac computerized tomography (CT) or magnetic resonance imaging (MRI). The echocardiography can assess parameters with proven prognostic value, such as LVH,21–23 LA dilation24 and the presence of valve calcification.25–27
The main objective of this study is to characterize the presence of valve calcification and its progression after 2 years in a population with CKD without known CVD. Another objective is to study the influence of non-classical risk factors on valvular calcification and to investigate the possible relationship with other manifestations of CVD such as carotid intima-media thickness (IMT), the presence of atheroma plaques in carotid and femoral and the ankle-brachial index (ABI).
MethodsThe present work was performed in patients included in the NEFRONA28 study which is an observational, multicenter study, in patients with CKD stages 3 to 5D. It was designed to learn, during a 4 years follow, about the development of atheromatous disease and its predictive value on cardiovascular morbidity and mortality. The complete protocol of the study as well as the inclusion and exclusion criteria have been published.28,29 Since the completion of recruitment (June 2011), the data obtained have been used by other researchers who had presented a project and had been approved by the Scientific Committee.
An specific research proposal was presented and accepted in March 2016 to study the echocardiograms performed in the protocol that had not been analyzed to date. The use of the study database was authorized after signing the letter of commitment, yielding subsequently the echocardiographic variables obtained. Therefore, in the present study, the carotid and femoral atheromatosis and the presence of structural cardiac alterations in the echocardiogram were evaluated retrospectively together with the epidemiological and analytical data at the baseline visit and at 24 months.
The proposal included the analysis of 400 patients (800 echocardiograms) with a distribution by CKD stage similar to that of the initial study: Stage 2: 0.5% (2.5 patients), Stage 3: 38.4% (192 patients), Stage 4: 25% (125 patients), Stage 5: 8% (40 patients) Stage 5D: 28.1% (140.5 patients). The sample size was agreed with the principal investigators taking into account the time period for analysis with the initial objective of obtaining results within 2 years. Selection of patients was done at random at each stage of CKD and was carried out by the principal investigators. Patients whose studies were performed within the first 6 months of acquisition were excluded to avoid errors due to the learning curve of the technicians, and only those patients with a control echocardiogram at 24 months were included. All patients signed an informed consent and the study was approved by the Ethics Committee of the Arnau de Vilanova University Hospital (Lleida).
In the analysis of the echocardiograms, the following measures were performed:
- •
Degree of LVH, quantified as millimeters (mm) of interventricular septum (IVS) in parasternal long axis plane and, if image quality made it possible by, as absolute left ventricular (LV) mass indexed by body surface according to Mosteller's formula: body surface = √ body weight (kg) × height (cm)/3600). The measure of the indexed mass is the most adequate and reliable and it would be taken whenever it was available. The breakpoints for IVS and mass VI were taken from the Guide Quantification Chambers, and the formula for calculating the mass of LV: Mass of LV = 0.8 ⋅1.04 ⋅ {( IVS + LVEDD + PWd)3 − LVEDD3}} + 0.6 gr.30IVS = interventricular septum thickness, LVEDD = left ventricular end-diastolic diameter, PWd = LV posterior wall thickness in diastole. gr = grams.
- •
LV geometry as a function of end-diastolic dimension (LVEDD), relative wall thickness (RWT) and LV mass. The RWT is calculated with the following formula: (2 × LVPWd/LVDD are classified into: normal pattern (normal mass and GPR < 0.42), concentric remodeling (normal mass and RWT > 0.42), eccentric hypertrophy (mass and increased RWT < 0.42), concentric hypertrophy (increased mass and RWT > 0.42).
- •
LA dimensions (antero-posterior diameter in parasternal long axis plane in absolute centimeters (cm) and indexed by body surface according to Mosteller's formula. Measurement and cut-off points in accordance with current recommendations.30
- •
The presence/absence of valvular calcifications (grading of mitral and aortic calcification in mild/moderate/severe as well as the location of calcification in the case of mitral, annulus, leaflet, subvalvular or all).
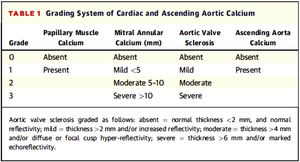
To assess valvular calcification and its grading, it was decided to respect the classification of valvular calcification pre-established in the NEFRONA study database (described below), which did not include a description of how the grading should be carried out. Since there is no standardized method in the literature, there were considered previous publications on valvular calcification assessment by echocardiography and finally it was decided to use the classification proposed by Gaibazzi et al. (2015); they proposed a scorefor the assessment of cardiac calcium (Fig. 1) and were able to demonstrate its independent association with cardiovascular events in patients undergoing stress echocardiography for clinical indication.31
Grading system for valvular calcification and ascending aorta of Gaibazzi et al.31.
Classification of valve calcification in the NEFRONA project:
- •
Presence of aortic calcification: yes/no.
- •
Gradation of aortic calcification: mild/moderate/severe.
- •
Presence of mitral calcification: yes/no
- •
Mitral calcification gradation: mild/moderate/severe.
- •
Type of mitral calcification: annulus/veil/subvalvular/all.
As shown in Fig. 1, mild mitral calcification was considered <5 mm, moderate 5–10 mm and severe >10 mm. Mild aortic calcification was considered as thickening >2 mm or increased echogenicity, moderate >4 mm and severe >6 mm.
To ensure the reproducibility of the measurements, 20 studies were randomly selected and the following measurements were repeated: IVS in mm, LVEDD in cm, PWd in mm, and the presence of aortic and mitral calcification was assessed with yes or no. For numerical variables, an analysis was carried out using an intraclass correlation coefficient that turned out to be 0.85. In the categorical variables, the Kappa Index was found to be 0.8.
Statistical analysisFor the processing and analysis of the data, we had the collaboration of the Biostatistics and Epidemiology Platform of the Health Research Institute of the Principality of Asturias (ISPA). The analysis was carried out using the R statistical package in version 3.5.
Quantitative variables with normal distribution are presented as mean (standard deviation) and the rest as median (interquartile range). Discrete variables are expressed as percentage. The relationships between discrete variables were assessed using Mc Nemar's chi-square. The comparison between continuous variables from two related groups was carried out with a Student's t analysis.
The analysis of the factors associated with valve calcification was performed using linear regression models when the dependent variable was continuous and logistic regression models when the dependent variable was binary. The covariates with showing p < 0.2 in the univariate analysis were included in the multivariate models. The Odds Ratio (OR) or relative risk coefficients and (RR) confidence intervals (CI) of 95% and associated p-values are reported. It is considered a statistically significant f if the p < 0.05 with a 95% CI. The Akaike information criterion (AIC) was used to choose the best final model.
ResultsEchocardiographic data from 397 patients were collected. All of them were extracted from the NEFRONA Study database after complying with the previously mentioned requirements regarding the acquisition of echocardiogram images. These are patients with a mean age at baseline visit of 59.1 ± 11.5 years, the majority male (61%) and white (97.7%).
The main characteristics in relation to CVRF, laboratory variables and vascular disease at the baseline visit and at 24 months are presented in Table 1. A high percentage of patients had HTN at the beginning of the study (91.4%) without further increase in the follow-up. More than a quarter of the patients were diabetic and almost three quarters had dyslipidemia at baseline, the proportion of both conditions increased at 24 months. The BMI was high illustrating overweight. Mean levels of LDL were initially 106 mg/dL and decrease significantly to 100 mg/dL, in parallel with an increase in percentage of patients treated with statins (63% at the start and 67% to 24 months), the change did not reach statistical significance, p = 0.082. A baseline, the mean serum concentration of iPTH were 118 pg/mL and increased to 146 pg/mL at 24 months, likely related to the progression of secondary hyperparathyroidism in patients with CKD, with an increase in serum phosphate that did not reached statistical significance, in the case of a trend (p = 0.195). Regarding vascular disease, it should be highlighted an increase in the area of carotid and femoral atherosclerotic plaque without appreciating a change in ABI or total IMT.
Characteristics of the population, baseline and at 24 months.
| Baseline visit | Visit at 24 month | P value | |
|---|---|---|---|
| HTA | 91.4% | 92.2% | 0.07 |
| Tobacco | Ex-smoker 38% | Former smoker 41.3% | 0.01 |
| Smoker 19.1% | Smoker 16.1% | ||
| Never smoked 42.6% | Never smoked 42.6% | ||
| DM | 28% | 30% | 0.013 |
| DL | 73.6% | 77.8% | <0.001 |
| BMI (kg/m2) | 28.7 ± 5.02 | 28.7 ± 5.07 | 0.92 |
| Abdominal circumference (cm) | 98.77 ± 12.48 | 100.4 ± 12.52 | 0.92 |
| Pulse pressure (PP) | 59.5 ± 15.97 | 59.47 ± 16.48 | 0.92 |
| eGFR (mL/min) | 33.24 ± 34.08 | 30.93 ± 18.00 | <0.001 |
| HDL mg/dL | 49.02 ± 13.79 | 50.42 ± 15.10 | 0.214 |
| LDL mg/dL | 106.03 ± 34.16 | 100.62 ± 32.26 | 0.023 |
| Total calcium mg/dL* uncorrected | 9.42 ± 0.55 | 9.42 ± 0.56 | 0.878 |
| P m /dL | 3.80 ± 0.95 | 3.88 ± 1.09 | 0.195 |
| CRP us mg/dL | 3.93 ± 8.22 | not available | |
| PTH i pg/mL | 118.36 ± 125.67 | 146.17 ± 117.75 | 0.009 |
| Albumin g/dL | 4.09 ± 0.56 | 4.03 ± 0.67 | 0.124 |
| Albuminuria mg/g | 167.36 ± 328.66 | 289.41 ± 591.81 | 0.065 |
| ABI | 1.06 ± 0.23 | 1.07 ± 0.26 | 0.386 |
| Total femoral plate area (mm2) | 0.75 ± 0.61 | 0.89 ± 0.69 | <0.001 |
| Total carotid plate area (mm2) | 0.37 ± 0.33 | 0.48 ± 0.48 | <0.001 |
| Sum total area (mm2) | 1.28 ± 0.85 | 1.51 ± 1.05 | <0.001 |
| IMT | 0.74 ± 0.14 | 0.75 ± 0.14 | 0.293 |
Comparison of CVRF, laboratory variables and vascular disease at the baseline visit and at 24 months with P values. BMI = body mass index. HTA = arterial hypertension, DM = diabetes mellitus, DL = dyslipidemia, eGFR = estimated glomerular filtration, ABI = ankle- brachial index, IMT = intima-media thickness.
The most frequent causes of nephropathy collected at the baseline visit were vascular disease (22%), glomerular nephropathy (15%) and diabetic nephropathy (13.6%), therefore vascular and metabolic disease were highly represented. The mean GFR, using the MDRD-4 equation (Modification of Diet in Renal Disease-4), at the beginning of the follow-up was 33.2 ml/min/1.73 m2 with a significant decrease at 24 months 30.9 ml/min/1.73 m, indicating progression of CKD during this 24 month period (Table 1).
Valve calcificationAt the beginning of the study, 30% of the patients had calcification of the aortic valve; Ttroughout the follow-up period, the number of patients with calcification increased to 43.1% (Table 2). The increase in calcification all was observed in all categories (mild, moderate and severe) (Appendix B see Table 1 in Supplementary material).
Evolution of aortic valve calcification.
| Baseline visit% | 24 month visit % | |
|---|---|---|
| Yes | 30 (119) | 43.1 (171) |
| Not | 63.7 (253) | 49.1 (195) |
| Missing values | 6.3 (25) | 7.8 (31) |
Presence of aortic valve calcification expressed as a percentage and absolute value (in parentheses) at the baseline visit and at 24 months.
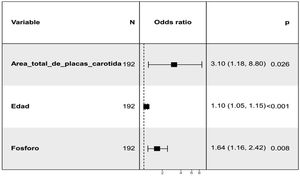
The relationship of aortic valve calcification at 24 months with the presence of potential risk factors at baseline visit was assessed. Thus, an initial unadjusted analysis was performed that showed a positive association with total carotid plaque area, total mean IMT, age, MD, serum phosphate (P) levels, and pulse pressure (PP). The use of Vitamin D and analogues (calcitriol, paricalcitol, alfacalcidol, cholecalciferol, calcifediol) as well as the use of calcium-based P binders were taken into account without having obtained a statistically significant association (Appendix B Table 2 in Supplementary material). In relation to LV growth patterns, there was a non-significant (p = 0.1) association with a concentric remodeling of LV (Appendix B see Fig. 1 of Supplementary material). Subsequently, a multivariate analysis was performed (Fig. 2) in which the positive relationship was maintained with the total carotid plaque area, P levels and age at the baseline visit. Regarding ABI, there is a trend that does not reach significance towards a higher ABI at the baseline visit in those with aortic valve calcification at 24 months.
Regarding the mitral valve, 24.2% of the patients had calcification at this level at the beginning of the study, increasing to 31% at 24 months (Table 3). There was an increase in all categories (ring, leaflets, subvalvular) (Appendix B, Tables 3 and 4 of Supplementary material).
Evolution of mitral valve calcification.
| Baseline visit% | 24 month visit % | |
|---|---|---|
| Yes | 24.2 (96) | 31 (123) |
| Not | 71 (282) | 61.2 (243) |
| Missing values | 4.8 (19) | 7.8 (31) |
Representation in percentage and in absolute numbers (in parentheses) of the presence of mitral valve calcification at the baseline visit and at 24 months.
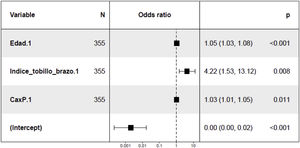
In the study of predisposing factors to mitral calcification at 24 months, the unadjusted analysis revealed a direct relationship with age, ABI, BMI, Ca x P product and PP at baseline (Appendix B Table 5 of Supplementary material). A trend towards an association with the presence of LV concentric hypertrophy was also observed at the baseline visit (p = 0.1) (Appendix B, Fig. 2 of Supplementary material). In the multivariate analysis (Fig. 3) the positive association with age, ABI and with the Ca x P product was maintained at the baseline visit.
DiscussionThis work is part of the NEFRONA project29 and has been performed to contribute to the previous publications of the study with the analysis of valvular calcification and its evolution. The most relevant finding is to substantiate the progression at 24 months of valve calcification, both aortic and mitral, in patients without a history of CVD and the association with different risk profiles.
It should be noted that there are no studies to date that demonstrate that the attenuation of the progression of vascular and/or valve calcification reduces the morbidity and mortality of patients with CKD. On the other hand, the correlation of its presence with CV events and hospitalization is established, demonstrating the usefulness of early detection even in patients in earlier stages of the disease.32
The analysis of the changes in the echocardiographic images at 24 months of follow-up, showed an increase in aortic and mitral calcification, reaching a prevalence of 43.1% and 31%, respectively. These findings indicate a progression of asymptomatic valve calcification, since these are patients with no history of CV events, with a prevalence of more than a third of the sample. Valvular function assessment was not included in the NEFRONA study protocol, but given the increase in the prevalence of aortic and mitral degeneration at 24 months, it can assume greater valve dysfunction and therefore an increased risk of heart failure and worsening of its vital prognosis. In addition, this work is novel in that it relates, in patients without previous CVD, cardiac structural alterations with other manifestations of vascular disease such as the appearance of atherosclerosis plaques in the carotid and femoral territory and their magnitude (measured as area in mm2), the IMT and the ITB.
To evaluate the probability of developing valve calcification, and thus to intensify the follow-up, we analyzed the relationship between valve calcification at 24 months and baseline variables visit that were considered potential markers or risk factors.
With respect to aortic valve calcification, it was observed that patients with LV concentric remodeling at the baseline visit have a greater tendency (which does not reach significance) to present aortic valve calcification at 24 months as compared to the rest of the LV geometric patterns. This finding was already described by Elmariah et al.33 in 2012, they observed that once adjusted for covariates such as hypertension, inflammatory factors (high-sensitivity CRP, interleukin-6) and subclinical vascular disease (interpreted as coronary calcification) there was an association between mitral and aortic calcification and LVH (especially concentric), unrelated to the obstructive valve gradient. These were also patients with no known CVD, but unlike our study it was not a cohort with CKD and the LV mass measurements were performed by MRI and valvular calcium assessment by CT. These techniques are more accurate of the measurements, but also increases the cost and time invested. The authors argue that the cause of this association could be unexplored parameters of inflammation or a truly undiagnosed hypertension.33 Unlike the aforementioned study, we observed a trend towards an association of calcification with the concentric remodeling pattern, a prior step in the alteration of the LV geometry to concentric hypertrophy, but ultimately it indicates the same underlying pathology. The fact that significance was not reached was probably due to the limitation of the sample size.
In the multivariate analysis performed to assess the relationship of aortic valve calcification at 24 months with baseline variables, once adjusted for the different covariates, it was observed a positive association with age, total carotid plaque area and P levels. Regarding age, it is widely recognized as one of the main risk factors for calcific aortic stenosis.34,35 It is described the association of elevated P levels with vascular calcification and endothelial dysfunction36,37 with increased risk of atherosclerosis and hypertension38 that may cause rupture of atherosclerotic plaque.39 In other publications of the NEFRONA study, the effect of serum P levels in the presence of subclinical atherosclerosis was analyzed using vascular ultrasound (carotid and femoral).40 Regarding the association with the total area of carotid plaque, it may indicate a significant relationship of aortic valve calcification with atherosclerotic disease in addition to the alteration of bone-mineral metabolism. There are no specific previous studies linking carotid atherosclerosis plaques with aortic valve calcification in patients with CKD, however there are publications indicating that there are pathophysiological mechanism that could explain such a relationship. Data suggest a similarity between aortic valve degeneration and atherosclerosis.41 It has been observed the presence of hemorrhage in the aortic leaflets similar to intraplate hemorrhage in the development of atherosclerosis and this finding has been related to a rapid progression of aortic stenosis. The onset of valve degeneration is related to endothelial dysfunction, local inflammation, and lipid deposition, while its progression is associated with mechanical stress, genetic factors, and the process of calcinosis. As described by Kleinauskienė et al.,41 the process begins on the vascular side of the leaflets with focal subendocardial lesions, similar to atherosclerosis plaques. Mechanical stress activates the cells of the vascular interstitium, inducing proliferation and mineralization, activating myofibroblasts and osteoblasts. Likewise, endothelial damage due to hemodynamic stress favors the deposition of LDL and lipoprotein A, particles with cytotoxic and inflammatory potential that also favor the initiation of mineralization. In fact, observational studies shows that statin treatment appeared to slow the progression of aortic valve degeneration, but this beneficial was not confirmed in prospective, randomized trials. In the univariate analysis, there was a statistically significant association of calcification with the product Ca x P with a p = 0.05, which was not maintained in the multivariate analysis, however the relationship with P remained significant. If the sample size had been was larger, both variables would have been included in the final model.
Previous studies such as CRIC18 obtained similar correlation between aortic valve calcification (measured by CT) and the reduction GFR in patients with CKD. This relationship was maintained independently of classic CVRF but it was lost if emerging risk factors were included (such as CRP and homocysteine). Therefore, these results also point towards the existence of other responsible factors.
Mitral valve calcification increased at 24 months in patients with concentric LVH at baseline, as previously described Elmariah et al.33 The analysis of mitral calcification at 24 months adjusted for the different covariates, showed that more calcification is observed in older patients, increased ABI and greater Ca x P product, thus assuming worsening of valve function, and consequently more cardiovascular morbidity. Age is a non-modifiable risk factor,42 but the control of the Ca x P product and the active search for subclinical atherosclerosis through the use of ABI helps to predict which patients will have a greater progression of mitral calcification. There are no previous studies with a specific design to show the association of ABI with valvular calcification, but there are a large number of references in the literature in patients with CKD that relate arterial stiffness with alterations in Ca and P metabolism and deposition of minerals in the arterial tunica media (with special involvement of the lower limbs),43 a deposit that also occurs at the valve level.44 As for the Ca x P product, it is a previously described risk factor with effects on vascular calcification (due to the deposition of hydroxyapatite crystals).45 It has also been found in previous publications association increased circulating levels of Ca and P with the calcification of the aortic valve.45
In the CRIC study cohort, mitral calcification was also assessed by CT in CKD (not on dialysis or transplantated).46 The presence of calcification of the mitral annulus was found to be independently related to age, Caucasian race, decreased GFR, and hyperphosphatemia. The prevalence of mitral annulus calcification in this population was 19.8% in Caucasian patients. In the present study, the prevalence of mitral valve calcification is higher (24.2% at baseline and 31% at 24 months), probably because there are also taken into account the calcifications at the level of the leaflets and subvalvular, and in addition these are patients with more advanced CKD (mean estimated GFR in CRIC was 41 ml/min our patients had our worked or 30–31 ml/min) and included dialysis patients.
LimitationsThe main limitations of the study are related to the retrospective type of analysis, the sample size and the quality of the echocardiographic image. This is a retrospective analysis of the data base, with the inherent limitations such as possible information bias. Regarding the sample size, 397 patients of the 400 expected were studied, interpretable results have been obtained consistent with previous research.
To minimize the problem of image quality, patients whose baseline study corresponded to the first 6 months of echocardiogram acquisition were excluded to avoid errors due to the learning curve. But image quality is also affected by the patient's echocardiographic window, a limitation inherent to any ultrasound technique. The exclusion of these patients could have affected the final results since only complete cases were included in the analysis of the multivariate models. Despite this, we have obtained models concordant with previous research and new data has been provided.
It should be taken into account that the gold standard for the evaluation of calcification is CT, but that the evaluation by echocardiogram is recommended due to the absence of radiation, portability and the possibility of valvular function evaluation.
ConclusionsThe present analysis of a sample of patients from the NEFRONA study shows that in individuals with CKD without known CVD there is a significant prevalence of subclinical cardiac valvular calcification. It was observed that after two years there is a significant progression of these lesions regardless of the GFR. This finding suggests the presence in these patients of specific risk factors that accompany the deterioration of renal function. Patients with a higher risk of mitral valve calcification at 2 years were identified as older, with subclinical peripheral vascular disease and alterations in bone-mineral metabolism. Patients with the highest risk of aortic valve calcification at 2 years are also older, with higher levels of P and with a greater total area of carotid plaque and a greater association with atherosclerosis. The identification of patients at higher risk could help to intensify medical treatment and follow-up, in order to avoid future CV events.
Conflicts of interestNone.
Please cite this article as: Martínez Fernández L, Sánchez-Alvarez JE, Morís de la Tassa C, Bande Fernández JJ, María V, Fernández E, et al. Factores de riesgo asociados a la calcificación valvular en pacientes con enfermedad renal crónica. Análisis del Estudio Nefrona. Nefrologia. 2021;41:337–346.