To the Editor,
Reversible posterior leukoencephalopathy syndrome (RPLS) involves altered vision and state of consciousness and convulsions, and is associated with several factors including uraemia, arterial hypertension (AHT), immunosuppressant treatment, and Goodpasture syndrome, among others. Here we present a case in which all of these conditions occurred simultaneously.
Our patient was a 22-year-old female who had developed severe renal failure for 4 months. Anti-MBG antibodies were positive, and we performed a renal biopsy that led to the diagnosis of extracapillary glomerulonephritis type I secondary to Goodpasture syndrome. She was treated with three 1g boluses of methylprednisolone, followed by prednisone at 60mg/day along with 9 sessions of plasmapheresis and oral cyclophosphamide (CP) at 1mg/kg/day. The patient did not develop pulmonary haemorrhage. She did not recover renal function and we decided to start haemodialysis therapy using a tunnelled central venous catheter. The patient’s overall health was good, although blood pressure remained high despite treatment with amlodipine, captopril, and atenolol.
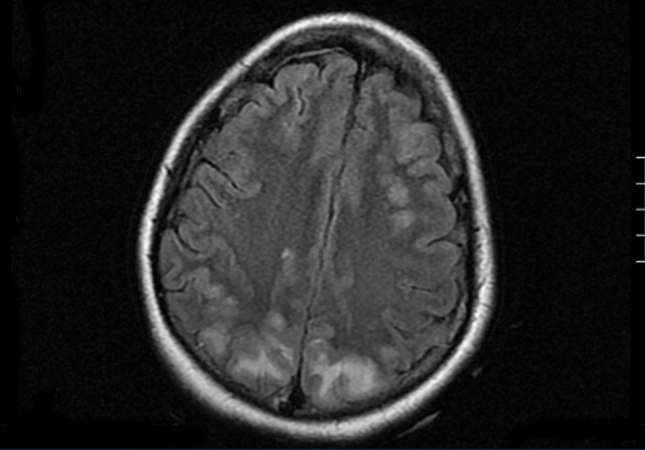
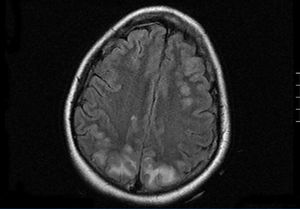
In the inter-dialytic period, the patient had general discomfort, temporary disorientation, and a fluctuating level of consciousness. She was referred to the emergency department, where she experienced two tonic-clonic seizures that were relieved with benzodiazepines, recovering consciousness between seizures. A physical examination revealed no fever, blood pressure (BP) of 151/108, spontaneous eye opening, and normal verbal responses. Pupils were isochoric and responded normally. Cranial nerve responses were normal, with preserved strength and sensitivity in all four limbs. Meningeal signs were negative, and no other findings of interest were observed. We performed a thoracic x-ray and cranial computed tomography with no relevant findings. With a third seizure and progressive worsening of general health, the patient was admitted to the intensive care unit. She needed sedation, endotracheal intubation, and induced mechanical ventilation (IMV). Laboratory analysis showed leukocytosis with a left shift, severe lactic acidosis, and negative anti-membrane antibodies, anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, immunocomplexes, anti-DNA, anti-SS-A, anti-SS-B, anti-RNP, and anti-Scl-70, with normal complement and immunoglobulin levels. Blood and urine cultures were negative, and ion levels were stable. Fibrobronchoscopy and lumbar puncture showed no findings. Cerebrospinal fluid cultures for bacteria, fungus, and viruses were negative. An electroencephalogram revealed a slow basal rhythm in the delta range with theta wave activity predominantly in both temporal regions. Two epileptiform discharges were observed in the right parietal region. A cranial nuclear magnetic resonance revealed signal hyperintensity in T2-weighted images and FLAIR sequences, suggesting vasogenic oedema, in the cortico-subcortical region of the posterior portion of both parietal, and occipital and right frontal lobes. The diffusion-weighted images showed no significant restriction (Figure). Given these findings, the patient was diagnosed with RPLS. Treatment was started with phenytoin, empirical antibiotic therapy, and continuous venovenous haemodiafiltration. The patient continued to experience severe AHT that required treatment with 6 different drugs (captopril, amlodipine, doxazosin, atenolol, nitroglycerin, and urapidil). In the following days, the patient improved considerably, with no new seizures and controlled blood pressure, which led to IMV withdrawal and discharge after 18 days of hospitalisation, with maintenance therapy based on atenolol and captopril.
DISCUSSION
RPLS1-3 involves vasogenic cerebral oedema that is generally associated with AHT, causing cerebral blood overflow and endothelial abnormalities and alters cerebral circulatory self-regulation. This disease mainly affects white matter due to its tissue density and the posterior lobules due to their scarce sympathetic innervation, making these regions more vulnerable to AHT. Several different causes have been proposed for this phenomenon (Table). It involves headache, altered state of consciousness, visual abnormalities (blurred vision, scotoma, cortical blindness) and tonic-clonic seizures. It is treated by controlling AHT, preferably using nicardipine or labetalol (precaution is recommended when using nitroprusside due to the possibility of triggering paradoxical cerebral vasodilation that would worsen the cerebral oedema), and seizures are prevented with phenytoin or benzodiazepines. The disease progresses in a relatively benign manner until recovery, although renal failure (uraemia and immunosuppression increase the neurotoxicity of hypertensive encephalopathy) and NMR results suggesting hyperintensity or extensive cerebral damage that affect the brain stem are poor prognostic markers.
In the medical literature, only two cases have been described of RPLS coexisting with Goodpasture syndrome. The first4 involved a 27-year-old male on treatment with CP and prednisone, with renal and pulmonary symptoms that arose during a hypertensive crisis that was resolved after 48 hours by controlling blood pressure and substituting CP for rituximab. The second5 was a 22-year-old female also with renal and pulmonary symptoms who was on haemodialysis and plasmapheresis treatment, not with CP. She needed IMV. In all three cases, symptoms were severe, with visual abnormalities, headache, and seizures, but were resolved in a maximum of 3 weeks with no sequelae or recurrences after controlling BP and, in the first case, after modifying the immunosuppressant treatment. In our case, the patient sought treatment 4 months after developing the disease, when her anti-MBG antibodies were negative and she was not taking any immunosuppressant therapy.
In this context, it is especially important to control AHT in order to prevent a severe set of symptoms that, although they generally progress benignly, may cause potentially severe encephalopathy.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Figure 1. Cranial nuclear magnetic resonance
Table 1. Causes of reversible posterior leukoencephalopathy syndrome