Respuesta a Domínguez Alpiñariz P.
Comentario en: Nefrologia 2013;33(6):849-50
Nefrologia 2013;33(3):389-99.
PMID: 23640095 [PubMed - in process]
Reply to Domínguez Alpiñariz P.
Comment in. Nefrologia 2013;33(6):849-50
Nefrologia 2013;33(3):389-99.
PMID: 23640095 [PubMed - in process]
To the Editor:
The authors reply to our publication on magnesium and chronic kidney disease1 and provide data on their preliminary experience with 10 peritoneal dialysis patients who received calcium acetate/magnesium carbonate (Osvaren®). The median treatment dose during follow-up was 2 pills per day (range 1-3). In two patients, it was necessary to reintroduce another phosphate binder at low doses. During follow-up, they only found one high magnesium value (1.8mmol/l) and none for calcium higher than 10.5mg/dl.
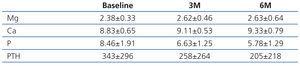
Firstly, it is necessary to highlight that the most important factor in serum magnesium concentrations is the concentration in dialysate, which the authors did not report. In our experience in peritoneal dialysis the mean magnesium concentrations with dialysate of 0.25mmol/l and of 0.50mmo/l were 2.04±0.3mg/dl (n: 17 patients) and 2.35±0.3mg/dl (n: 56 patients), respectively.2 We should bear in mind that, up to concentrations of <4.88mg/dl (<2.0mmol/l), hypermagnesaemia is clinically irrelevant and is associated with beneficial effects.1 The question that we must answer is whether in peritoneal dialysis patients who receive calcium acetate/magnesium carbonate (Osvaren®) these serum magnesium values increase above these figures. In our experience with 12 peritoneal dialysis patients (11 with dialysate of 0.50mmol/l and one with 0.25mmol/l) treated exclusively with calcium acetate/magnesium carbonate (Osvaren®) for 6 months, the mean serum magnesium values increased from 2.38±0.33 to 2.63±0.64, with the highest value reached in a patient being 3.5mg/dl (Table 1).
In the Calmag study3 on haemodialysis patients, the number of calcium acetate/magnesium carbonate pills (Osvaren®) required was 7.29±3.026/day, and as such, the dose the authors referred to in their letter (2 pills per day with a range from 1 to 3) is rather low, which may explain why in two patients, it was necessary to reintroduce another phosphate binder. In our haemodialysis studies over six months in real clinical practice (n: 52 patients), the mean CaMg dose required for the reduction of phosphorus (from 6.43±1.93 at baseline to 4.83±1.98mg/dl after six months) was 4.66±1.52 pills without the requirement for any other phosphate binder.4
Therefore, we agree with the authors of this letter that calcium acetate/magnesium carbonate (Osvaren®) has an important role as the first step of treatment in peritoneal dialysis and that it is important to carry out more comprehensive studies in peritoneal dialysis patients.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Biochemical parameters in peritoneal dialysis patients treated with Ca-Mg