The best way to comply with funding policies is to declare the entity that has funded your research study and the grant that you have received. We found no reference to the funding of your research study in your manuscript. Please confirm that this is correct. If you would like to declare an entity that has funded your research, please specify that entity's full name and country as well as the IDs of the grant in the text of your article.
Chronic kidney disease (CKD) is currently considered a pandemic disease affecting approximately 10% of the adult population throughout the world.1 In Mexico, the incidence and prevalence of CKD requiring replacement therapy has been calculated to be 466 cases per million people (pmp) and 1409pmp, respectively.2
Kidney transplant (KT) is undoubtedly the therapy of choice for these patients. According to information from the National Transplant Centre (Centro Nacional de Trasplantes, CENATRA), 12,741 patients are on the waiting list for KT, of which it is estimated that 30% are sensitised.3,4
In about 30% of patients, the potential donor is incompatible with the recipient, either due to incompatibility of the blood group (ABO) or to immunological incompatibility. The natural alternative for recipients who are incompatible with their potential donors is to add them to the waiting list to receive a cadaveric transplant.
Due to the permanent increase in patients with chronic kidney disease requiring replacement therapy, candidates for transplantation and the concomitant shortage of organs, alternatives for incompatible pairs have been proposed, such as paired, chained or sequential KT, in order to increase the number of patients that may benefit from KT.
The purpose of this report is to describe the first 12 months of post-transplant evolution of recipient patients who participated in the first KT chain carried out in Mexico.
We reviewed the list of renal failure patients in replacement therapy who had initiated the protocol for KT at INCMNSZ and had been incompatible with their potential living donors. The procedure was explained to them and they were asked to sign the informed consent form. After histocompatibility tests were performed and patients had been assigned using a computer programme developed for this event, a total of 4 chain donation transplants were performed.
Different combinations of potential chains were obtained from a population of 20 non-compatible pairs, starting with the “altruistic” donor and ending with a recipient on the waiting list.
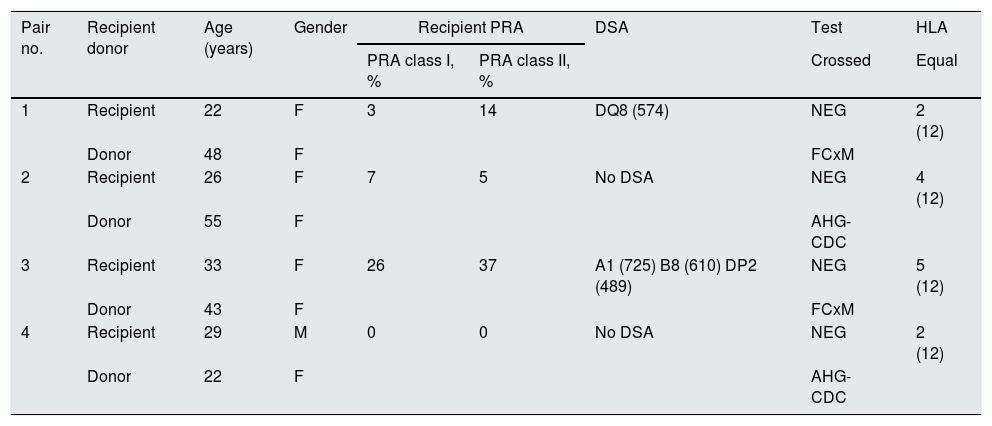
Table 1 shows the clinical, demographic and immunological characteristics of the patients. All recipients received triple immunosuppression treatment based on tacrolimus, mycophenolate mofetil and prednisone according to the dose and blood levels stipulated in the institutional protocol; all received induction therapy. All 4 transplants took place without surgical complications in donors and recipients; the 4 recipients showed immediate graft function.
Demographic and immunological characteristics.
| Pair no. | Recipient donor | Age (years) | Gender | Recipient PRA | DSA | Test | HLA | |
|---|---|---|---|---|---|---|---|---|
| PRA class I, % | PRA class II, % | Crossed | Equal | |||||
| 1 | Recipient | 22 | F | 3 | 14 | DQ8 (574) | NEG | 2 (12) |
| Donor | 48 | F | FCxM | |||||
| 2 | Recipient | 26 | F | 7 | 5 | No DSA | NEG | 4 (12) |
| Donor | 55 | F | AHG-CDC | |||||
| 3 | Recipient | 33 | F | 26 | 37 | A1 (725) B8 (610) DP2 (489) | NEG | 5 (12) |
| Donor | 43 | F | FCxM | |||||
| 4 | Recipient | 29 | M | 0 | 0 | No DSA | NEG | 2 (12) |
| Donor | 22 | F | AHG-CDC | |||||
AHG-CDC: anti-human globulin-enhanced complement-dependent cytotoxicity crossmatch; DSA: donor specific antibodies; FCxM: flow cytometry crossmatch; HLA: human leukocyte antigens; PRA: panel reactive antibody.
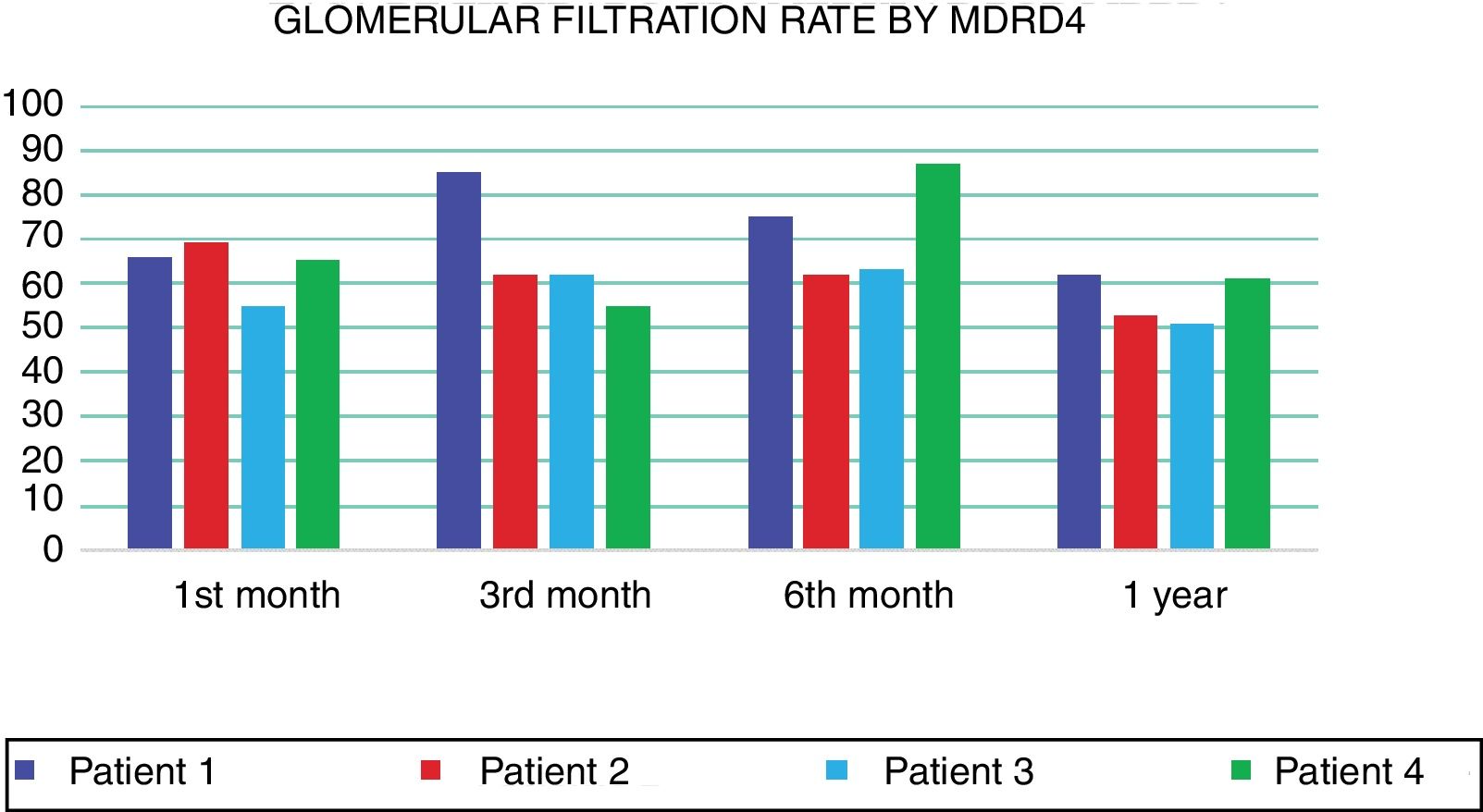
At 12 months, all recipients are alive with good renal function, observing good glomerular filtration rates (GFR) as estimated by MDRD equation (Fig. 1). In terms of immunological events, there were 2 acute rejections mediated by antibodies. In both patients, these rejections were managed using the current standard treatment, which includes plasmapheresis, intravenous immunoglobulin, rituximab and bortezomib. After the treatment, immunological reaction disappeared as verified in later biopsies.
It has been shown that chain KT is a good alternative for patients who have a healthy but incompatible living donor. It is also widely known that the number of kidney grafts from cadaveric donors is insufficient, so that being able to carry out chain transplants by finding the best living donor for each participating recipient helps reduce the number of patients waiting for a deceased donor organ, and allows the participating recipients to shorten their time on dialysis, with the consequent benefits.
The KT chain is a simpler and less expensive method for overcoming incompatibility than desensitisation protocols, which involve not only the inherent costs of desensitisation but also those derived from the post-transplant follow-up and constant laboratory histocompatibility tests.5 Without a doubt, KT chains not only represent a great biological benefit for the patient, but also a decrease in costs for health systems.4
We know that the probability of finding suitable pairs will depend on the number of participants, and undoubtedly a national list could facilitate the identification of compatible pairs and the practice of many more transplants.
The experience of this first KT chain in our centre and in Mexico has demonstrated that this valuable alternative is feasible and successful in our country, and is of benefit to patients.
Please cite this article as: Martínez Calderón P, Cruz Martínez R, Parmentier de León C, Grimaldo Rico OE, Castelán Carmona N, Madrigal Bustamante JA, et al. Reporte de la primera cadena de trasplante renal en México. Nefrologia. 2019;39:452–453.