Renal replacement therapies (RRT) as support for acute kidney injury in critically ill patients have become a routine and essential practice in their management, resulting in the widespread use of various techniques among these patients, such as intermittent hemodialysis (IHD), extended hemodialysis and continuous RRT (CRRT).
In this review we aim to summarize current evidence of indication, choice of modality, timing of initiation, dosing and technical aspects of RRT. We carried out a narrative review based on guidelines, consensus documents by main working groups and the latest relevant clinical trials on RRT in the critically ill.
We did not find enough evidence of any RRT modality having superior benefits in terms of patient survival, length of intensive care unit/hospital stay or renal outcomes among critically ill patients, in spite of optimization of clinical indication, modality, timing of initiation and intensity of initial therapy. This is still a controverted matter, since only early start of high-flux CRRT has been proven beneficial over IHD among hemodynamically unstable postoperative patients.
Our objective is to portrait current RRT practices in multidisciplinary management of critically ill patients by intensive care and nephrology professionals. Implication of a nephrologist in the assessment of hemodynamic status, coexisting medical conditions, renal outcome expectations and management of resources could potentially have benefits at the time of RRT selection and troubleshooting.
Las terapias de reemplazo renal (TRR) para el abordaje del fracaso renal agudo (FRA) de los pacientes inestables en la unidad de cuidados intensivos (UCI) se han convertido en una medida rutinaria e imprescindible para su manejo de tal manera que, tanto la hemodiálisis intermitente (HD), como las formas híbridas (HD extendida) o continuas (TRR continua) pueden emplearse indistintamente en estos enfermos. Con esta revisión pretendemos resumir de forma ordenada la evidencia disponible en cuanto a indicación, selección de modalidad, momento de inicio, dosificación y aspectos técnicos de las TRR.
Hemos realizado una revisión narrativa a partir de las guías vigentes, documentos de consenso de los principales grupos de trabajo y últimos ensayos clínicos relevantes sobre la TRR.
En nuestra revisión no hemos encontrado evidencia de que ninguna modalidad de TRR prescrita en pacientes en UCI obtenga beneficios tangibles en términos de supervivencia, estancia en UCI/hospitalización ni recuperación de la función renal; a pesar de su optimización en cuanto a indicaciones, selección de modalidad, momento y/o intensidad de inicio de la técnica. Es más, en la literatura actual todavía existe controversia sobre la superioridad de una modalidad de TRR sobre otra ya que, sólo en los pacientes post- quirúrgicos hemodinámicamente inestables se ha podido demostrar un beneficio al emplearse una TRR continua de alto flujo e inicio precoz frente a una HD.
Con la evidencia actual pormenorizada en nuestra revisión pretendemos poner de manifiesto la tendencia actual al manejo multidisciplinar por intensivistas y nefrólogos de estas terapias en UCI, lo cual podría reportar beneficios en la evolución clínica de los enfermos críticos y dar cabida a que el punto de vista del nefrólogo se tuviera en cuenta de manera rutinaria en la toma de decisiones sobre el estado hemodinámico, las condiciones médicas coexistentes, la disponibilidad recursos y el posible efecto sobre la función renal a largo plazo a la hora de seleccionar y gestionar los problemas de cada modalidad de TRR seleccionada.
Acute renal failure (AKI) is a common heterogeneous disorder in critically ill patients hospitalized in intensive care units (ICU) that is associated to significant morbidity and mortality at short- and long – term.1 Many authors propose prevention as the best treatment, optimizing the state of hydration, electrolyte homeostasis and avoiding the use of nephrotoxic agents.1,2 Approximately 5–10% of patients with established AKI require renal replacement therapy (RRT) during their ICU admission. Studies carried out by the Spanish intensive care medicine services3,4 estimate that the prevalence of AKI in ICU is 42.4%, and RRT is needed in 38% of cases and the associated mortality is 29.7%. However, there is large variability between different studies due to variability in patient profiles (medical and surgical ICUs), definitions of AKI and multiple treatment modalities.2
Risk factors for the use of RRT include advanced age, male gender, severity of the underlying disease, sepsis, decompensated heart failure, cardiac surgery, liver failure, and the use of mechanical ventilation.5 The indication of RRT increases a 10% per year, perhaps due to the change of the critical patient profile, is increasingly elderly, comorbid and undergoing complex surgery.5 Therefore, RRT, previously considered an extraordinary measure in the ICU for patients in need of dialysis and hemodynamic instability, has become a routine technique in the management to these patients.
In Spain, nephrologists were highly involved in the early days of RRT in the ICUs. They develop technical adaptations based on their experience in conventional intermittent hemodialysis (HD). However, the management of continuous RRT has been disappearing from the basic curriculum of the training of the average nephrologist. Only 4 of the 24 nephrology services of the Madrid region continue to be involved in the complete management of AKI in the ICU. In the rest there is a sequential follow-up in which continuous RRT depends on the intensivists, and in the nephrologist begins to intervene when the patient is changed to HD or goes to the conventional ward. Most studies, reviews and recent experience on the fundamentals issues of RRT come from the daily work of the anesthesiologist or ICU doctors. However, the working groups of the scientific societies in this field do include intensivists and nephrologists.6,7
Recent studies published in our country recommend close preventive monitoring and nephroprotection of critically ill patients with comorbidity.8 It seems interesting to design joint protocols that foresee the intervention of the nephrologist in the complete follow-up process: prevention, RRT in ICU, switch to conventional dialysis with follow-up in the ward, and up to the recovery of kidney function.
In this review, we intend to summarize the available evidence and provide a multidisciplinary view of the management of critical patients undergoing continuous RRT and/or HD to be used as a guide by the nephrologist on everyday clinical practice. Will try to clarify who needs treatment, when to start, which regimen or modality is optimal, and how to step down the treatment.
We have carried out a narrative review based on current guidelines, consensus documents from the main working groups and the latest relevant clinical trials on RRT in the ICU. We conducted a bibliographic search for MesH [Acute Kidney Injury] and [Critical Care] terms in MEDLINE OVID SP, EMBASE OVID SP and PUBMED selecting clinical trials and reviews between 2009 and 2019. In addition, the Cochrane Kidney and Transplant Specialized Register was consulted, Cochrane Central Register of Controlled Trials (CENTRAL).
Selection of renal replacement therapy modality (which)There is controversy over which is the optimal RRT modality for patients with AKI in the ICU. The selection of the initial modality is frequently based on the availability of resources, the experience of each center, and the tolerance which is conditioned by the hemodynamic status of the patient. Additionally, transitions between various modalities are frequent due to changes in the patient's clinical situation and specific complications of the technique such as coagulation of the system.
The Kidney Disease: Improving Global Outcomes (KDIGO)6 guidelines recommend the following RRT modalities in critically ill patients: HD, continuous RRT, and prolonged intermittent therapies (a hybrid of the prior two modalities). The Acute Disease Quality Initiative (ADQI) Workgroup7 insists that it is important to know the functions and mechanisms of each technique that define the advantages and disadvantages for its use in each situation.
By prescribing HD, it is achieved a rapid diffusive clearanceof small molecules with relatively short treatment (3−5 h), conditioning the ultrafiltration rate (UF) to the patient’s hemodynamic tolerance. The continuous RRT, will provide a more gradual elimination of fluids and solutes by convective clearance of larger molecules during a long period of time (optimally 24 h per day). Hybrid HD modalities are characterized by treatments that generally last between 8−16 h, with intermediate rates of UF and clearance.6–9
Therefore, the theoretical advantages of continuous RRT over HD are based on its slow and progressive process, which would lead to greater hemodynamic stability, better control of the water and electrolyte balance, improvement in microcirculation due to the preferential elimination of interstitial fluid, flexibility to adapt treatment to the specific needs of the patient at all times and easy clinical monitoring of therapy. The drawbacks include the need for immobilization and an increase in cost as compared to HD. Hypothermia, another of the classic disadvantages with significant added risks for the patient (loss of energy, chills, increased oxygen demand, vasoconstriction, immunosuppression, arrhythmias, decreased cardiac contractility, tissue hypoxia and coagulation disorders) can be remedied today with the use of temperature control systems, such as line heaters or air blankets.7–9
By contrast, the greater purifying efficacy of HD makes it more recommended for cases of severe hypercalcemia or hyperkalemia, some acute poisonings and tumor lysis syndromes. Its short duration allows time for early mobilization and rehabilitation, as well as for other diagnostic and therapeutic interventions without the need to interrupt therapy.7–9
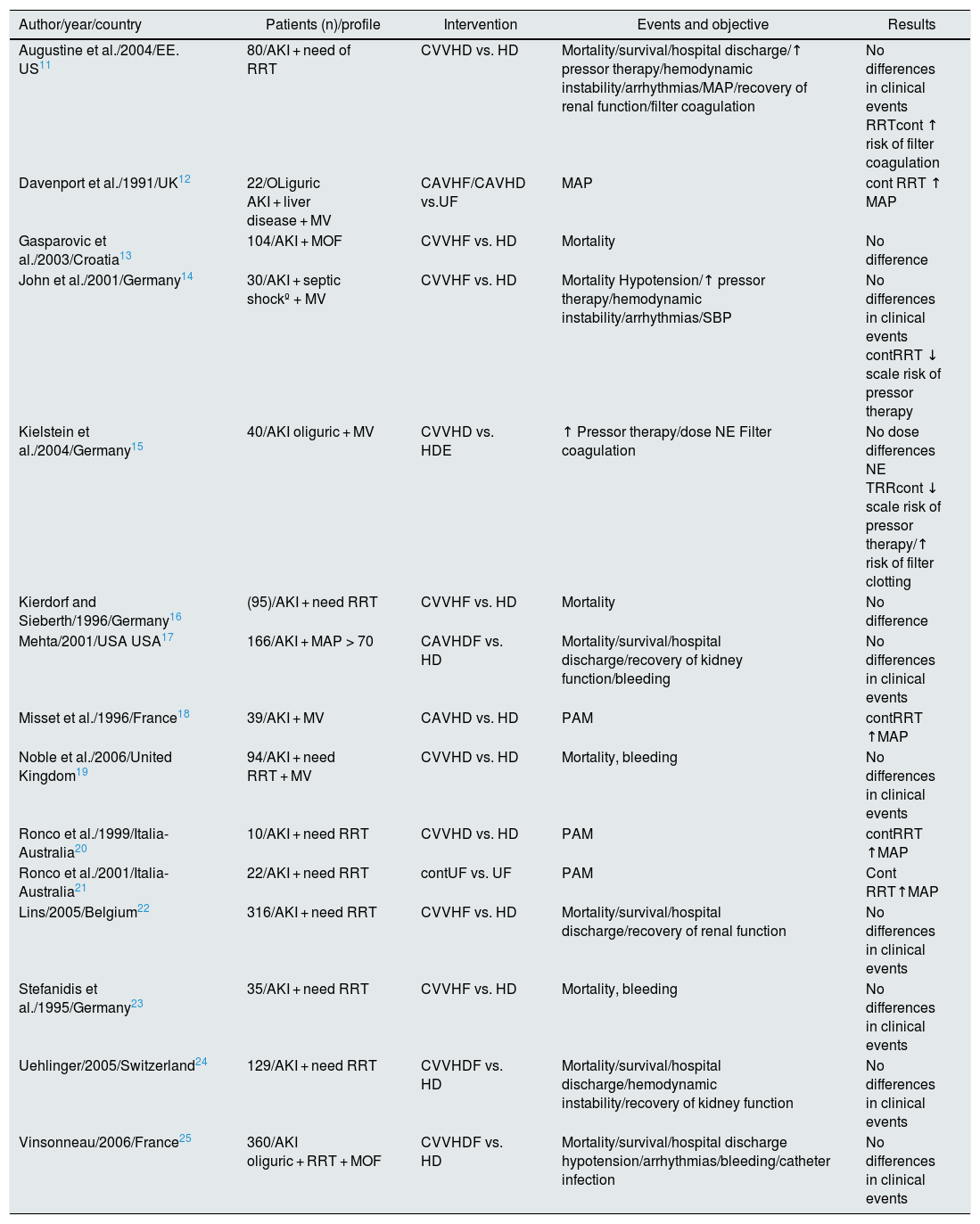
However, the systematic review published by the Cochrane Library in 200710 is unable to demonstrate significant advantages for any of the procedures after pooling the 15 randomized clinical trials (RCT) available at that time11–25 (Table 1). The authors concluded that neither of the 2 techniques (continuous HD or RRT) is superior to the other in risk of mortality in ICU/hospitalization, length of stay in ICU/hospitalization, recovery of renal function after AKI and/or cardiovascular stability (defined by hypotension, systolic blood pressure, dose of inotropic drugs, and risk of bleeding). Furthermore, a greater tendency to filter coagulation is evidenced in those treated with continuous RRT. These studies have some limitations to be integrated into a meta-analysis, since the definitions of AKI are not the same, there is a high rate of interchange between RRT modalities and there are differences in technical aspects, such as the type and dose of anticoagulation system.
Comparison of randomized clinical trials for mortality.
| Author/year/country | Patients (n)/profile | Intervention | Events and objective | Results |
|---|---|---|---|---|
| Augustine et al./2004/EE. US11 | 80/AKI + need of RRT | CVVHD vs. HD | Mortality/survival/hospital discharge/↑ pressor therapy/hemodynamic instability/arrhythmias/MAP/recovery of renal function/filter coagulation | No differences in clinical events RRTcont ↑ risk of filter coagulation |
| Davenport et al./1991/UK12 | 22/OLiguric AKI + liver disease + MV | CAVHF/CAVHD vs.UF | MAP | cont RRT ↑ MAP |
| Gasparovic et al./2003/Croatia13 | 104/AKI + MOF | CVVHF vs. HD | Mortality | No difference |
| John et al./2001/Germany14 | 30/AKI + septic shockº + MV | CVVHF vs. HD | Mortality Hypotension/↑ pressor therapy/hemodynamic instability/arrhythmias/SBP | No differences in clinical events contRRT ↓ scale risk of pressor therapy |
| Kielstein et al./2004/Germany15 | 40/AKI oliguric + MV | CVVHD vs. HDE | ↑ Pressor therapy/dose NE Filter coagulation | No dose differences NE TRRcont ↓ scale risk of pressor therapy/↑ risk of filter clotting |
| Kierdorf and Sieberth/1996/Germany16 | (95)/AKI + need RRT | CVVHF vs. HD | Mortality | No difference |
| Mehta/2001/USA USA17 | 166/AKI + MAP > 70 | CAVHDF vs. HD | Mortality/survival/hospital discharge/recovery of kidney function/bleeding | No differences in clinical events |
| Misset et al./1996/France18 | 39/AKI + MV | CAVHD vs. HD | PAM | contRRT ↑MAP |
| Noble et al./2006/United Kingdom19 | 94/AKI + need RRT + MV | CVVHD vs. HD | Mortality, bleeding | No differences in clinical events |
| Ronco et al./1999/Italia-Australia20 | 10/AKI + need RRT | CVVHD vs. HD | PAM | contRRT ↑MAP |
| Ronco et al./2001/Italia-Australia21 | 22/AKI + need RRT | contUF vs. UF | PAM | Cont RRT↑MAP |
| Lins/2005/Belgium22 | 316/AKI + need RRT | CVVHF vs. HD | Mortality/survival/hospital discharge/recovery of renal function | No differences in clinical events |
| Stefanidis et al./1995/Germany23 | 35/AKI + need RRT | CVVHF vs. HD | Mortality, bleeding | No differences in clinical events |
| Uehlinger/2005/Switzerland24 | 129/AKI + need RRT | CVVHDF vs. HD | Mortality/survival/hospital discharge/hemodynamic instability/recovery of kidney function | No differences in clinical events |
| Vinsonneau/2006/France25 | 360/AKI oliguric + RRT + MOF | CVVHDF vs. HD | Mortality/survival/hospital discharge hypotension/arrhythmias/bleeding/catheter infection | No differences in clinical events |
AKI: acute kidney injury ; MOF, multi-organ failure; HD: intermittent hemodialysis; CAVHD: continuous arteriovenous hemodialysis; CVVHD: continuous venovenous hemodialysis; CAVHDF: continuous arteriovenous hemodiafiltration; CVVHDF: continuous veno-venous hemodiafiltration; CAVHF: continuous arteriovenous hemofiltration; CVVHF: continuous veno-venous hemofiltration; NE: norepinephrine; MAP: mean arterial pressure; SBP: systolic blood pressure; RRT: renal replacement therapy; cont RRT: continuous renal replacement therapy; UF: intermittent hemofiltration; cont UF: continuous slow hemofiltration ; MV: mechanical ventilation.
Source: Cochrane Library 2007.10
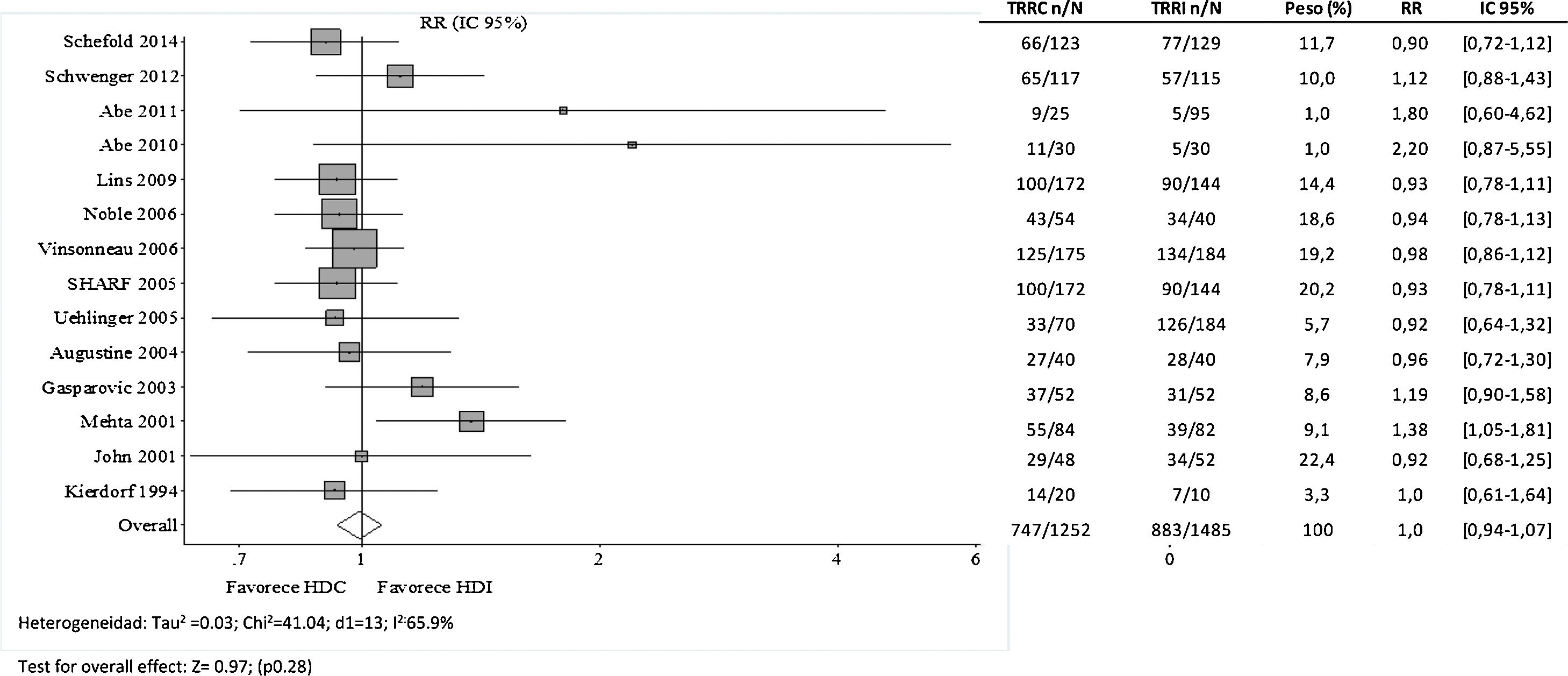
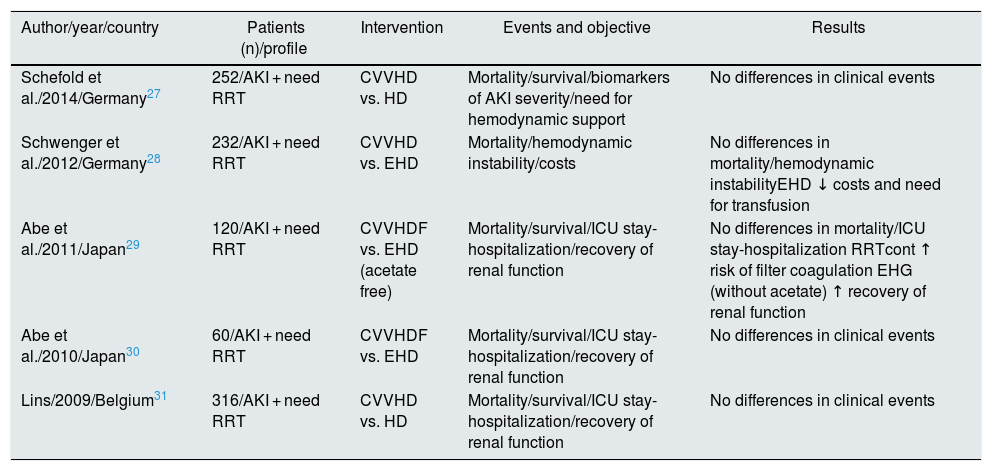
It might be thought that the RCTs previously listed in Table 1 (prior to 2008) do not reflect technical advances or the current profile of critical patients with AKI. Therefore, we have combined these studies with those published by Schoenfelder et al.26 in 2017 (Table 2). As shown in Fig. 1, the 5 RCTs27–31 added in the new meta-analysis reach the same conclusion, and no favorable trend is observed with respect to the older ones. However, the observational studies collected in this same work do point out that continuous RRT is more effective than HD in achieving a well-tolerated negative fluid balance,32,33 improving hemodynamic support and expanding the indication for RRT in critically ill patients.
Comparison of randomized clinical trials.
| Author/year/country | Patients (n)/profile | Intervention | Events and objective | Results |
|---|---|---|---|---|
| Schefold et al./2014/Germany27 | 252/AKI + need RRT | CVVHD vs. HD | Mortality/survival/biomarkers of AKI severity/need for hemodynamic support | No differences in clinical events |
| Schwenger et al./2012/Germany28 | 232/AKI + need RRT | CVVHD vs. EHD | Mortality/hemodynamic instability/costs | No differences in mortality/hemodynamic instabilityEHD ↓ costs and need for transfusion |
| Abe et al./2011/Japan29 | 120/AKI + need RRT | CVVHDF vs. EHD (acetate free) | Mortality/survival/ICU stay-hospitalization/recovery of renal function | No differences in mortality/ICU stay-hospitalization RRTcont ↑ risk of filter coagulation EHG (without acetate) ↑ recovery of renal function |
| Abe et al./2010/Japan30 | 60/AKI + need RRT | CVVHDF vs. EHD | Mortality/survival/ICU stay-hospitalization/recovery of renal function | No differences in clinical events |
| Lins/2009/Belgium31 | 316/AKI + need RRT | CVVHD vs. HD | Mortality/survival/ICU stay-hospitalization/recovery of renal function | No differences in clinical events |
AKI: acute renal failure; HD: intermittent hemodialysis; EHD: extended hemodialysis; CVVHD: continuous venovenous hemodialysis; CVVHDF: continuous venovenous hemodiafiltration; CVVHF: continuous venovenous hemofiltration; RRT: renal replacement therapy; contTRR: continuous renal replacement therapy; ICU: intensive care unit.
Forest plot of intervention studies comparing continuous hemodialysis (CHD) vs. hemodialysis (HD) in terms of mortality risk (Integrated from Cochrane 2007 and Schoenfelder 2017).
Foot: CHD: continuous hemodialysis; IHD: intermittent hemodialysis; CRRT: continuous renal replacement therapy; IRRT, intermittent renal replacement therapy; RR: relative risk; CI: 95% Confidence Interval.
Another future potential strategy to be considered when optimizing fluid management and maintaining hemodynamic stability in patients with RRT would be that proposed by Tumlin et al. in its publication in 2018.34 This study shows that the vasoactive treatment with angiotensin II would improve the recovery of kidney function and limit the need for long-term RRT in critically ill patients with AKI. This publication has been recently reinforced after the approval by the Food and Drug Administration35 of the prescription of angiotensin II in the treatment of distributive shock, since its administration increases the mean arterial pressure with less deleterious effects (such as water retention at the renal level), as compared to a great variety of other vasopressors able to modulate the sympathetic system and with a direct vasoconstrictor effect on smooth muscle (dopamine, felinephrine and vasopressin).
Along the same lines, Nash et al.36 published in 2017 another meta-analysis that analyzes the evolution of critical patients with AKI, treated with 3 types of techniques: continuous RRT, extended HD and conventional HD. The authors did not find significant advantages in any of them, either in in-hospital mortality, or in length of stay. It is likely that the 3 may be more indicated in one specific stage of the patient's evolution. It would be necessary to look for a specific study design that includes the temporal sequence of use of RRT and with a longer follow-up able to analyze the effect beyond admission, including mid-term outpatient follow-up on the recovery of kidney function or need for regular dialysis.
None of this new evidence allows us to recommend a single type of RRT for all critical patients in need of RRT, and we cannot go beyond what is recommended by the KDIGO guidelines,6 the consensus document of the ADQI group7 and the guidelines for the Spanish Society of Nephrology.37 Clinicians must know the pros and cons of each technique and adapt it to the clinical characteristics of the individual, the type of AKI, its potentially reversible causes, the resources of their center (including availability and cost) and experience.38 In this scenario, nephrologists could contribute to the early detection of renal damage, risk stratification, the nephroprotective prevention and a sequence of RRT appropriate to each case, as recommended by the guidelines National Institute for Health and Care Excellence.39
Indications for renal replacement therapy (to whom)Creatinine levels and glomerular filtration rate are considered crucial to define the severity of AKI and the need to start RRT. Initially, the Consensus Conference of the ADQI group in 200440 defined the Risk Injury Failure (RIFLE) method with 3 levels of acute renal dysfunction based on an increase in Cr and a reduction in urine output. This classification has been surpassed after the publication of the consensus between intensivists and nephrologists of the Acute Kidney Injury Network (AKIN),41 which has been accepted because it has greater specificity than the RIFLE.
Due to the reduction of glomerular filtration, the AKI patients may develop volume overload, electrolyte abnormalities, metabolic acidosis and/or uremic symptoms.1 The indication of RRT is based on these 3 key abnormalities.
It is common for non-oliguric patients to present a volume overload due to the inability of the kidney to eliminate the massive supply of serum therapy, parenteral nutrition, blood products and intravenous medications required in critical situations. There is no a threshold of volume overload that determines the initiation of RRT, but several observational studies find an association between the amount of volume overload at the beginning of RRT and mortality in the ICU,42,43 so many recommend initiating RRT in situations of overload refractory to diuretics that compromise the function of a vital organ (increased cardiac afterload, ascites, respiratory distress or increased intrarenal pressure).
The effect of the UF rate on the survival of critically ill patients undergoing CVVHDF is controversial. Slow UF maintains the situation of edema and tissue dysfunction for longer period of time, but a rapid extraction of fluid generates a stress that is difficult to manage by the critical patient. While some studies recommend high rates of UF > 25 ml/kg/day to improve the patient,44 others find opposed results. For example, in the subanalysis by tertiles of the RENAL study carried out on 1,434 patients from 34 ICUs, it was shown that the risk of mortality at 90 days was higher in patients receiving a UF of 1.75 ml/kg/h than those who 1.01 received ml/kg/h (i.e., 25 ml/kg/day).45 The authors themselves recognize that there are confounding factors; it is an uncontrolled observational study, the UF rate was left to the preference of the clinician, thus it cannot be determined whether the elevated UF is harmful in itself or simply reflects patients with more volume greater with worse hemodynamic status that required a higher UF.
We have certain strategies to improve tolerance to high rates of UF in HD in chronic patients. For example, the ESHOL46 study describes how the management of biosensors, UF/sodium profiles, and prescription of on-line pre/post-dilution hemofiltration during the routine course of dialysis sessions improves tolerance in chronic ambulatory patients. The results of the ESHOL study cannot be extrapolated at all to critical patients with AKI, but they could indicate new strategies to improve hemodynamic status in a situation of high UF.
Metabolic acidosis is part of the AKI, so we can set a threshold for starting the RRT in cases with a pH < 7.1–7.2 or bicarbonate level < 12−15 mmol/l.1,47 Severe hyperkalaemia (serum K > 6.5 mmol/l) resistant to medical treatment carries a risk of cardiotoxicity and arrhythmias, therefore it requires early initiation of RRT. For these 2 situations (acidosis and hyperkalemia), HD is more effective than continuous RRT.6,47 However, in the case of severe hyponatremia in the context of AKI, the continuous RRT allows a slow and controlled correction of serum sodium and prevents neurological sequelae by demyelination.47 Likewise, the use of RRT is indicated to alleviate the symptoms and effects of uremia (> 100−110 mg/dl) favoring platelet dysfunction, malnutrition/anorexia, heart failure and greater susceptibility to infection and sepsis.47–50
Finally, several studies propose continuous RRT with a high convective rate as a complement in the management of sepsis in ICUs to eliminate proinflammatory cytokines. However, the reviewed trials have not shown a significant benefit over the usual HD approach, even when high replacement doses are used in continuous RRT.49,50 Although RRT increases lactate clearance, there is little evidence that its initiation for this reason alone modifies the clinical course in patients with severe lactic acidosis without associated drug toxicity (as in the case of metformin).51
After reviewing the available literature, we recommend that RRT should be started in all patients with AKIN III AKI with clear symptoms of fluid overload (acute lung edema, heart failure, platelet dysfunction, intestinal congestion and refractory edema despite the use of diuretics). and/or in those cases in which uremic symptoms, ionic alterations or acid-base balance entail a vital risk because they compromise the correct functioning of the main organs.
Initiation of renal replacement therapy (when)There is no protocol that defines when to start RRT in an uniform manner between countries, centers or even between intensivists and nephrologists in the same center. As defined by KDIGO6 the onset of RRT is generally established with an AKIN III stage.41 Some situations may recommend its early initiation, even without renal dysfunction. These are: critical electrolyte alterations, fluid overload or poisonings. In these cases, the risks inherent to the technique such as vascular access complications (hemorrhage, thrombosis, vascular injury or infection), intradialytic hypotension and ionic imbalance (hypocalcemia, hypophosphataemia or hypokalaemia) must be weighed.
For more than a decade, an attempt has been made to establish recommendations to assess the best time to start RRT. Bagshaw et al.52 proposed an algorithm based on absolute indications, RIFLE grade and non-renal indications. In Spain, Leoz et al.53 published a guide with recommendations for the initiation and choice of the most appropriate modality of RRT in the ICU.
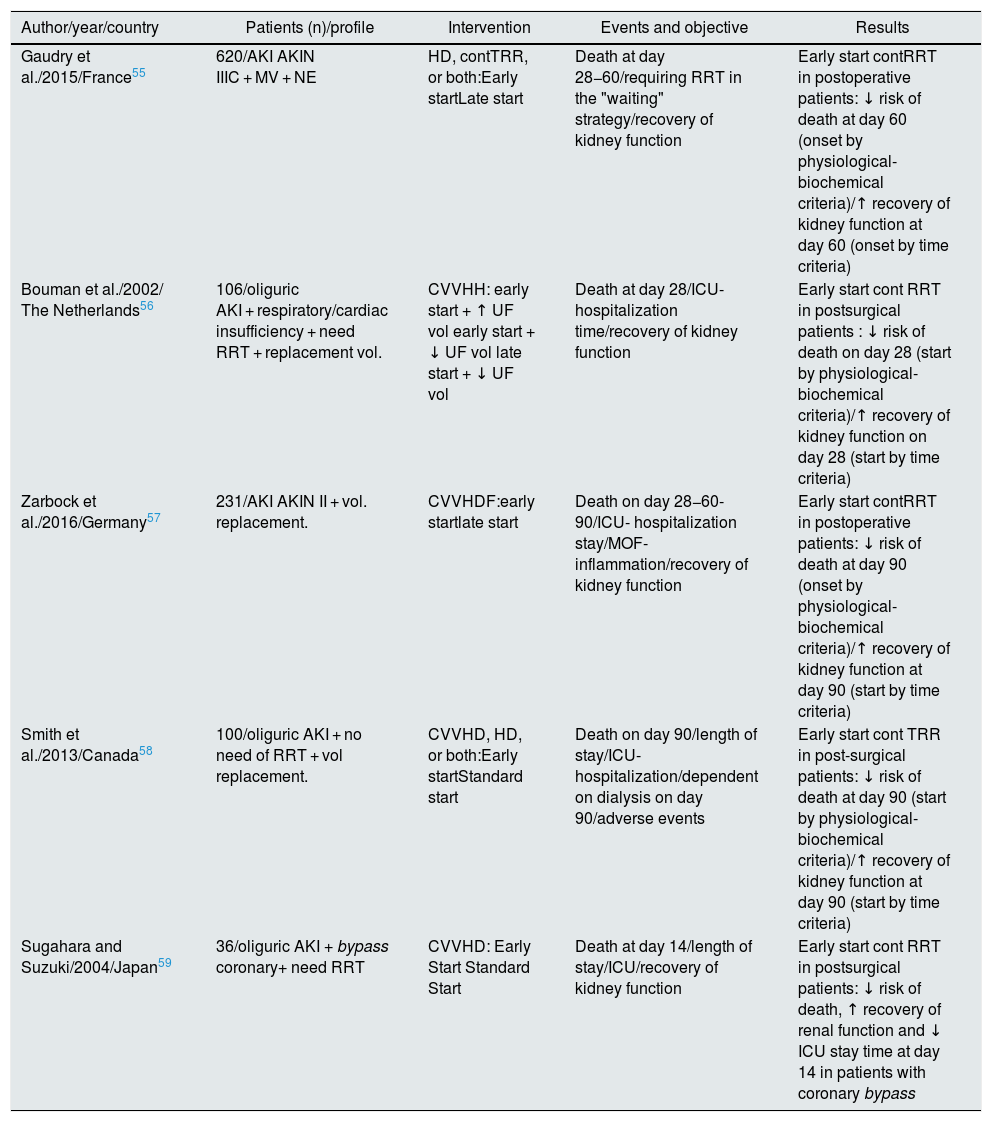
It is worth highlighting a meta-analysis from the Cochrane Library published in 201854 that addresses the specific question of when to start RRT and with what technique55–59 (Table 3). It shows a certain benefit for the early initiation of RRT with a favorable trend in reduction of mortality, stay ICU and renal recovery. However, in the stratified analysis, these benefits are restricted to the subgroup of critical postoperative patients always treated with continuous RRT. On the other hand, they describe an increase in the incidence of adverse effects related to the early initiation of RRT, for which they conclude that the level of evidence should be considered moderate-low.
Comparison of randomized clinical trials for early vs. late start.
| Author/year/country | Patients (n)/profile | Intervention | Events and objective | Results |
|---|---|---|---|---|
| Gaudry et al./2015/France55 | 620/AKI AKIN IIIC + MV + NE | HD, contTRR, or both:Early startLate start | Death at day 28−60/requiring RRT in the "waiting" strategy/recovery of kidney function | Early start contRRT in postoperative patients: ↓ risk of death at day 60 (onset by physiological-biochemical criteria)/↑ recovery of kidney function at day 60 (onset by time criteria) |
| Bouman et al./2002/ The Netherlands56 | 106/oliguric AKI + respiratory/cardiac insufficiency + need RRT + replacement vol. | CVVHH: early start + ↑ UF vol early start + ↓ UF vol late start + ↓ UF vol | Death at day 28/ICU-hospitalization time/recovery of kidney function | Early start cont RRT in postsurgical patients : ↓ risk of death on day 28 (start by physiological-biochemical criteria)/↑ recovery of kidney function on day 28 (start by time criteria) |
| Zarbock et al./2016/Germany57 | 231/AKI AKIN II + vol. replacement. | CVVHDF:early startlate start | Death on day 28−60-90/ICU- hospitalization stay/MOF-inflammation/recovery of kidney function | Early start contRRT in postoperative patients: ↓ risk of death at day 90 (onset by physiological-biochemical criteria)/↑ recovery of kidney function at day 90 (start by time criteria) |
| Smith et al./2013/Canada58 | 100/oliguric AKI + no need of RRT + vol replacement. | CVVHD, HD, or both:Early startStandard start | Death on day 90/length of stay/ICU-hospitalization/dependent on dialysis on day 90/adverse events | Early start cont TRR in post-surgical patients: ↓ risk of death at day 90 (start by physiological-biochemical criteria)/↑ recovery of kidney function at day 90 (start by time criteria) |
| Sugahara and Suzuki/2004/Japan59 | 36/oliguric AKI + bypass coronary+ need RRT | CVVHD: Early Start Standard Start | Death at day 14/length of stay/ICU/recovery of kidney function | Early start cont RRT in postsurgical patients: ↓ risk of death, ↑ recovery of renal function and ↓ ICU stay time at day 14 in patients with coronary bypass |
AKIN: Acute Kidney Injury Network; MOF, multi-organ failure; AKI: acute renal failure; HD: intermittent hemodialysis; CVVHDF: continuous venovenous hemodiafiltration ; CVVHD: continuous venovenous hemodialysis; CVVHF: continuous venovenous hemofiltration; NE: norepinephrine; RRT: renal replacement therapy; contTRR: continuous renal replacement therapy; ICU: intensive care unit; UF: intermittent hemofiltration; SUF: continuous slow hemofiltration; MV: mechanical ventilation.
Source: Cochrane Library 2018.54
It is also worth to mention that the most recent meta-analyzes published in 2017 by Lai et al.,60 Christiansen et al.61 and Zou et al.,62 that did not find clear benefits in the early initiation of RRT in terms of mortality, recovery of renal function or length of stay in the ICU; however, once again they reported a favorable trend in the subgroup of patients undergoing cardiac surgery with onset of RRT within the first 24 h post-surgery.
Another retrospective observational study by Jia et al.63 concludes that late initiation of RRT could be associated with a higher incidence of short-term mortality among critically ill patients in the ICU, and recommends early initiation of RRT in the presence of oligoanuria before the development of AKIN III; however, it should be taken into considaration the limitations, statistical power and retrospective design of this study as compared to those mentioned previously.
Therefore, after reviewing the trials and meta-analyzes presented in this section, an early start of continuous RRT could only be recommended in critically ill postoperative patients, since early prescription of this treatment could reduce the mortality rate and promote recovery of renal function in this type of patients.
Renal replacement therapy dose (how much)Taking into account the physicochemical bases previously mentioned, the clearance of urea and other low molecular weight solutes is a direct function of the effluent flow used in any RRT modality.1,6,7
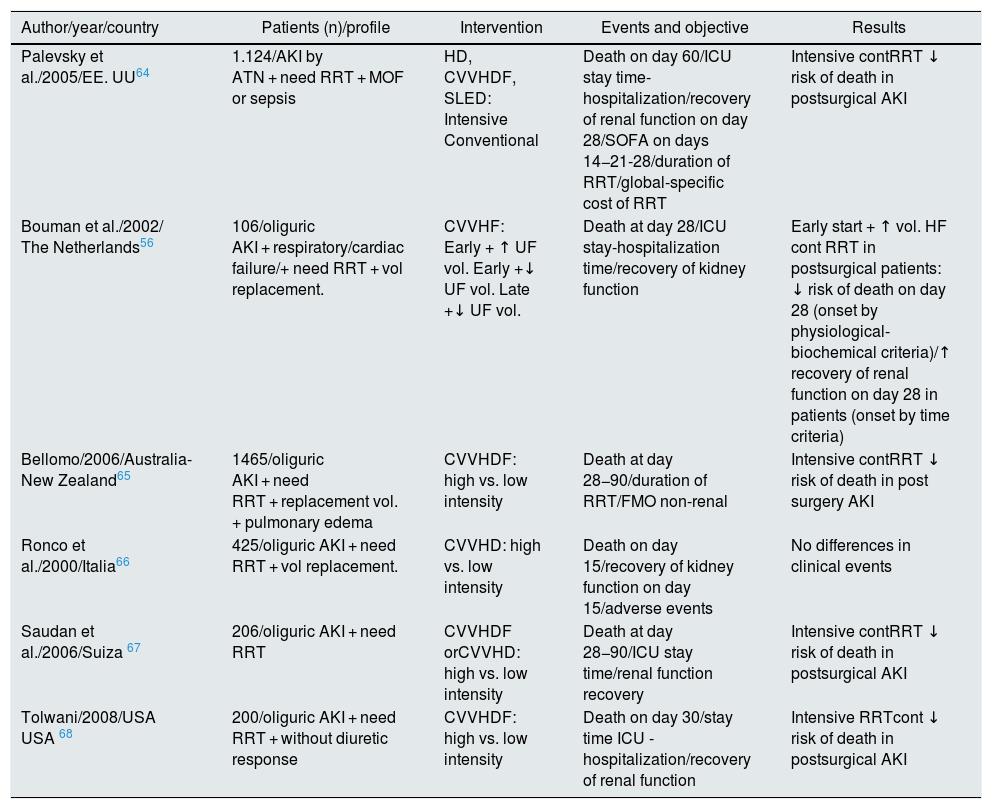
Several observational studies explored the effect of the dialysis dose and suggest that a high flow rate of effluent (> 35 ml/kg/h) is associated with improved survival in ICU.56,64–68 However, these conclusions have not been confirmed by subsequent systematic reviews, such as the 2016 Cochrane Library69 (Table 4). In none of the 6 controlled clinical trials included could be demonstrated that higher effluent flow doses produced significant benefits in: 30-day mortality, length of hospitalization e and/or recovery of renal function at medium/long term. Only in a specific subgroup of critically ill patients, postoperative patients, the risk of mortality is significantly reduced and the possibility of recovering kidney function is increased by using continuous high-flow RRT. Furthermore, in patients with fulminant liver failure or brain injury with elevated intracranial pressure, continuous high-dose RRT is also recommended, since it helps to maintain a better maintenance of cerebral perfusion,70,71 despite assuming the possibility of iatrogenesis due to «Dialytrauma» and the loss of certain beneficial cytokines in the initial management of sepsis commonly present in these patients.
Comparison of randomized clinical trials for dose intensity.
| Author/year/country | Patients (n)/profile | Intervention | Events and objective | Results |
|---|---|---|---|---|
| Palevsky et al./2005/EE. UU64 | 1.124/AKI by ATN + need RRT + MOF or sepsis | HD, CVVHDF, SLED: Intensive Conventional | Death on day 60/ICU stay time-hospitalization/recovery of renal function on day 28/SOFA on days 14−21-28/duration of RRT/global-specific cost of RRT | Intensive contRRT ↓ risk of death in postsurgical AKI |
| Bouman et al./2002/ The Netherlands56 | 106/oliguric AKI + respiratory/cardiac failure/+ need RRT + vol replacement. | CVVHF: Early + ↑ UF vol. Early +↓ UF vol. Late +↓ UF vol. | Death at day 28/ICU stay-hospitalization time/recovery of kidney function | Early start + ↑ vol. HF cont RRT in postsurgical patients: ↓ risk of death on day 28 (onset by physiological-biochemical criteria)/↑ recovery of renal function on day 28 in patients (onset by time criteria) |
| Bellomo/2006/Australia-New Zealand65 | 1465/oliguric AKI + need RRT + replacement vol. + pulmonary edema | CVVHDF: high vs. low intensity | Death at day 28−90/duration of RRT/FMO non-renal | Intensive contRRT ↓ risk of death in post surgery AKI |
| Ronco et al./2000/Italia66 | 425/oliguric AKI + need RRT + vol replacement. | CVVHD: high vs. low intensity | Death on day 15/recovery of kidney function on day 15/adverse events | No differences in clinical events |
| Saudan et al./2006/Suiza 67 | 206/oliguric AKI + need RRT | CVVHDF orCVVHD: high vs. low intensity | Death at day 28−90/ICU stay time/renal function recovery | Intensive contRRT ↓ risk of death in postsurgical AKI |
| Tolwani/2008/USA USA 68 | 200/oliguric AKI + need RRT + without diuretic response | CVVHDF: high vs. low intensity | Death on day 30/stay time ICU -hospitalization/recovery of renal function | Intensive RRTcont ↓ risk of death in postsurgical AKI |
AKI: acute renal failure; MOF, multi-organ failure; HD: intermittent hemodialysis; CAVHD: continuous arteriovenous hemodialysis; CAVHDF: continuous arteriovenous hemodiafiltration; CVVHDF: continuous veno-venous hemodiafiltration; CVVHD: continuous venovenous hemodialysis; HF: intermittent hemofiltration; CAVHF: continuous arteriovenous hemofiltration; CVVHF: continuous venovenous hemofiltration; NE: norepinephrine; ATN: acute tubular necrosis; MAP: mean arterial pressure; SCUF: slow continuous ultrafiltration; SLED: sustained low-efficiency dialysis ; SOFA: Sequential Organ Failure Assessment ; RRT: renal replacement therapy; cont RRT: continuous renal replacement therapy; IRRT: intermittent renal replacement therapy; MV: mechanical ventilation.
Source: Cochrane Library 2016.69
More recent published studies such as IVOIRE72 in 2013 and RESCUE73 in 2017, do not resolve the uncertainty, and conclude that not even very high fluxes of up to 70 ml/kg/h achieve an improvement in renal or patient survival. However, in the stratified subanalysis of RESCUE, favorable results were found for the AKI subgroup in burned patients with septic shock treated with high doses of RRT.73
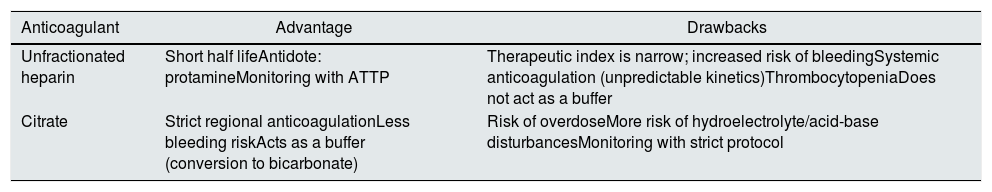
Kidney replacement therapy anticoagulation (how to do it)Coagulation of the extracorporeal circuit is the most common technical complication during continuous RRT given the fact that 30–60% of patients cannot be anticoagulated due to added risk of bleeding.74 The strategies to minimize the risk of coagulation of the circuit are the following: increase the blood flow rate, decrease the filtration fraction, ensure the optimal function of the catheter, balance the dose of UF/convection (alternating even the pre and post filter replacement) and increasing the frequency of pre-scheduled replacement of the extracorporeal circuit. All this depends on close control of the therapy with clinical and technical monitoring by a multidisciplinary team of specialized nurses, intensivists and nephrologists. Once the strategy has been optimized, the most commonly used anticoagulation therapies are based on heparin or citrate 74–78 (Table 5).
Advantages and disadvantages of each renal replacement therapy anticoagulation system.
| Anticoagulant | Advantage | Drawbacks |
|---|---|---|
| Unfractionated heparin | Short half lifeAntidote: protamineMonitoring with ATTP | Therapeutic index is narrow; increased risk of bleedingSystemic anticoagulation (unpredictable kinetics)ThrombocytopeniaDoes not act as a buffer |
| Citrate | Strict regional anticoagulationLess bleeding riskActs as a buffer (conversion to bicarbonate) | Risk of overdoseMore risk of hydroelectrolyte/acid-base disturbancesMonitoring with strict protocol |
APTT: activated thromboplastin time.
Regional citrate anticoagulation (RCA) has been used routinely for continuous RRT in critically ill patients for more than 25 years, and is recommended for patients with and without a contraindication to heparin.6,7,37 Recent evidence75 has confirmed the superiority of regional citrate compared to systemic anticoagulation with heparin, both in maintaining the patency of the extracorporeal circuit and in reducing bleeding complications.
The use of RCA in ICUs has been standardized, and concerns about its possible adverse effects and additional cost are avoided with the protocols and systems that are discussed below.
Citrate anticoagulation is based on its Ca chelating effect in the extracorporeal circuit, which inhibits the coagulation cascade. This happens when the Ca ion falls below 0.5 mmol/l and it is most effective below 0.25 mmol/l. Citrate is infused with a pump at the precircuit end of the RRT in proportion to the blood flow, so that its levels reach between 4–6 mmol/L, which is sufficient to reduce the level of ionic Ca in the circuit below 0.35 mmol, achieving inhibition of the coagulation cascade.76 Most RCA protocols require an infusion of calcium to maintain physiological levels of ionic Ca in the systemic circulation and achieve effective anticoagulation. Citrate and calcium infusion rates can be adjusted on most currently available machines, and infusion pumps are integrated and connected through software, thus there is no reason to be concerned about uncontrolled infusion rates.76 Likewise, there are new monitors of continuous RRT with systems, such as the Aquarius™ with integrated RCA, which allow the use of acid citrate dextrose solutions in the pre-blood pump instead of the classic trisodium citrate. These solutions, such as Prismocitrate® 10/2, containing 10 mmol/l citrate, 2 mmol/l of citric acid, in addition to sodium and chloride in physiological concentrations. Thus, the risk of hypernatremia and metabolic alkalosis is minimized, avoiding the electrolyte and acid-base alterations classically associated with RCA.75,76 Finally, the citrate-calcium complex dissociates in the systemic circulation and is rapidly metabolized to bicarbonate by the liver, serving as a beneficial alkalizing agent in patients with AKI and acidosis.77
Therefore, after reviewing the available evidence, we can conclude that RCA is associated with better circuit patency, lower risks of bleeding, and avoids heparin-induced thrombocytopenia.78 The prescription must be individualized and would benefit from close monitoring by a multidisciplinary team that ensures efficacy and minimizes complications.
Completion of renal replacement therapy (how long)The recovery of an adequate diuresis rhythm is usually the fundamental event to consider the withdrawal of RRT according to the recommendations of the KDIGO6 and ADQI group7 guidelines, although the normalization of the biochemistry parameters may take longer. For this reason, the possibility of definitively ending RRT is unlikely in real clinical practice; the transition to other modalities is considered advisable during the process of recovery of the critical patient.
Extended HD can be used as a bridge therapy between continuous techniques and HD if it is justified by the clinical condition of the patient. This aspect depends as much on the individual medical criteria as on the circumstances of the patient and the technology available.
The transition from continuous RRT to HD allows the beginning of physical recovery and the mobilization of the patient out of bed, a fundamental aspect of their evolution before being transferred to a hospital ward.79
Finally, it should not be forgotten that it is important to ensure a good quality of information for the patient, family members or legal guardians on the aspects involved in RRT and its short-term prognosis, from both, the intensive care service taking care of the patient, and the nephrology consultants who administer the RRT.
ConclusionsBoth AKI and volume overload are frequent complications of critically ill patients in the ICU that compromise their hemodynamic and respiratory status, resulting in increased morbidity and mortality. Many working groups have proposed to anticipate these factors with an almost preventive use of RRT, which does not have a clear scientific support in a generalized manner. Only in postoperative critical patients it has been possible to demonstrate a decrease in mortality and length of stay in the ICU when continuous high-flow RRT is prescribed early. Likewise, the tendency during the last decades to increase in the dose of dialysis has not reported clear benefits, only in this same subgroup of patients in whom the probability of recovery of kidney function at the long term has improved.
It is worth highlighting the evolution in the field of filter anticoagulation, the RCA is consolidating itself as the technique of choice when it comes to optimizing the permeability of the circuit, reducing the risk of bleeding and thrombocytopenia so common with the use of heparin classical. Today, monitors software greatly facilitates handling of citrate and calcium, and solutions with physiological ion concentrations minimize the metabolic risks associated with this form of anticoagulation.
Therefore, currently with any RRT modality (whether continuous, hybrid or intermittent) prescribed in an appropriate, individualized and even sequential way, we can be certain about a good tolerance despite the severe hemodynamic instability characteristic of these patients. Both intensivists and nephrologists involved in its management must know the pros and cons of each technique, thus personalized treatment protocols can be established and adapt them according to the clinical evolution of each individual.
In conclusion, it is worth recognizing that we still did not solve the question of when and how to finalize a treatment with RRT, this constitutes a challenge for future studies.
FinancingFunded by an “Unrestricted Grant” from the Iñigo Álvarez de Toledo Renal Foundation (FRIAT) through the Madrid Nephrology Foundation and the Segovia de Arana-Puerta de Hierro-Majadahonda Research Institute (018/02FRA).
Conflict of interestsThe authors of this article declare that there is no potential conflict of interest related to the article.
We are grateful for the technical assistance of Dr. Paula López Sánchez, PhD, in the methodological and analytical aspects and of Ms. Cristina Escudero Gómez in the bibliographic search.
Please cite this article as: Valdenebro M, Martín-Rodríguez L, Tarragón B, Sánchez-Briales P, Portolés J. Una visión nefrológica del tratamiento sustitutivo renal en el paciente crítico con fracaso renal agudo: horizonte 2020. Nefrologia. 2021;41:102–114.