The purpose of this case report is to describe the regression of vascular calcifications (VC) in a patient with secondary hyperparathyroidism (SHPT) after having added cinacalcet to her treatment. We present the clinical case of a 48-year-old woman with chronic renal failure secondary to tubulointerstitial disease. She was being treated with long-term haemodialysis (HD) and underwent two kidney transplants with transplantectomies. The patient presented with severe SHPT caused by parathyroid gland hypertrophy. The radiology test showed signs of VC in the radial and interdigital arteries, and VC in a linear arrangement were observed in both breasts on the mammography. Cinacalcet was added to her treatment with vitamin D derivatives and phosphate-binding agents, which resulted in a good control of mineral metabolism. The radiology test showed that the calcification in the interdigital artery had disappeared and that the bone appeared to be more structured. The mammography also showed regression of the VC. To conclude, cinacalcet may have potential for regression of VC in patients with SHPT.
El propósito de este informe de caso es describir la regresión de las calcificaciones vasculares (CV) en una paciente con hiperparatiroidismo secundario (HPTS) tras añadir cinacalcet a su tratamiento. Presentamos un caso clínico de una mujer de 48 años de edad con insuficiencia renal crónica secundaria a nefropatía túbulo-intersticial, tratada con hemodiálisis (HD) de larga duración y sometida a dos trasplantes renales con trasplantectomías. La paciente presentaba HPTS grave causado por la hipertrofia de la glándula paratiroidea; la radiología mostró signos de CV en las arterias radiales e interdigitales y la mamografía, CV lineales múltiples en ambas mamas. Se añadió cinacalcet al tratamiento previo con derivados de la vitamina D y agentes quelantes del fósforo, lo que dio como resultado un buen control del metabolismo mineral. La radiología mostró que las calcificaciones de la arteria interdigital habían desaparecido y que el hueso presentaba un aspecto más estructurado. La mamografía también mostró una regresión de las CV. En conclusión, cinacalcet puede tener potencial para la regresión de las CV en pacientes con HPTS.
INTRODUCTION
The presence of vascular calcifications (VC) are traditionally associated with chronic kidney disease (CKD); nevertheless, until recently it had been considered a passive phenomenon with little clinical importance. Over the last decade various epidemiological studies identified vascular calcification as an independent risk factor of cardiovascular mortality, in both the general and uraemic populations.1-5
Although the aetiopathogenic mechanisms of VC are not accurately defined and the factors related to its appearance are multiple, mineral and bone disorders are key factors in this process. Hyperphosphataemia, treatment with vitamin D, calcium salt overdose, hypercalcaemia episodes, remodeling bone disorders, etc., are direct causes of the serious VC load in patients with kidney problems.1-6
To date, VC has been considered an irreversible process, and nephrologists’ efforts had been channelled to slow down its progression.6,7 Although regression is unlikely, the use of therapeutic alternatives recently available and proper control of hyperparathyroidism (SHPT) have generated expectations.
Calcification of the mammary artery detected in the mammography is proof of generalised atherosclerotic vascular disease (ASVD), in both general population and diabetic patients. A mammography is a highly sensitive diagnosis technique for calcification characterisation, including VC; furthermore, it may be a potentially useful tool for diagnosing VC in CKD women.8
CASE REPORT
We present the case of a 48-year-old woman with chronic renal failure secondary to tubulointerstitial nephropathy. The patient had received long-term HD replacement treatment and undergone two kidney transplants with transplantectomies: the first one was due to acute humoral rejection and the second due to acute humoral and vascular rejection. She resumed haemodialysis in March 2005.
The intact-parathyroid hormone (iPTH) serum values were intermittently high. The patient had been treated previously with calcitriol for short periods of time, since it caused hypercalcaemia and hyperphosphataemia.
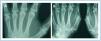
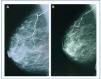
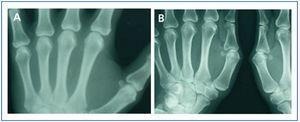
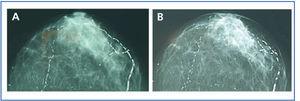
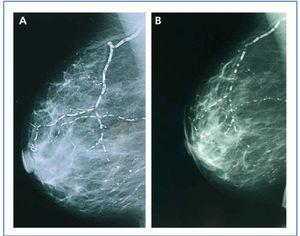
In January 2006, she presented serious SPTH, with notable increase in iPTH levels. The parathyroid ultrasound revealed an echo-rich pseudo-nodular structure in the posteriomedial area of the left thyroid lobe, compatible with parathyroid gland hypertrophy. A series of X-rays showed signs of hyperparathyroidism in the bones of both hands and wrists, with VC in radial and interdigital arteries. The mammography revealed multiple linear VC in both breasts (Figures: 1A, 2A, 3A and 4A; hand and mammary gland images in 2006).
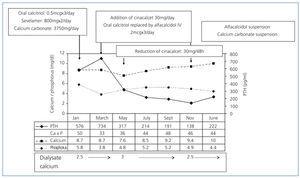
The patient was only treated with calcium carbonate initially. Later, oral calcitriol and sevelamer (800mg with main meals), a phosphate-binding agent (P), were added to the treatment and calcium carbonate dose was reduced. Figure 5 shows Ca, P and serum iPTH levels. Hyperphosphataemia was controlled after 2 months’ treatment and the Ca-P product was adequate; however, iPTH levels increased to 734pg/ml, so treatment was changed. Oral calcitriol was replaced with 30mg of oral cinacalcet once a day and intravenous alfacalcidol (2µg) was given immediately after haemodialysis. Calcium carbonate and sevelamer doses were not adjusted.
Two months later, the calcimimetic dose was reduced due to hypocalcaemia (7.6mg/dl). Intravenous vitamin D and calcium carbonate treatment was maintained, and the dialysate calcium changed from 2.5 to 3mEq/l.
During the following 6 months, all the parameter levels were maintained within the interval recommended by the KDOQI guidelines. In November 2006 excessive supression of iPTH (138pg/ml) and a potential hypercalcaemia (9.4mg/dl) were noticed, so treatment with calcium carbonate and alfacalcidol was suspended. In January 2007 treatment continued with sevelamer (800mg with main meals) and a minimum weekly dose of calcimimetic (30mg cinacalcet MMondays and Ffridays), resulting in good control of mineral and bone metabolism.
During this period, the X-ray series showed that the interdigital artery calcifications had disappeared, and the bone appeared better structured (Figures 1B and 2B; hand X-ray, 2007). The mammography also showed regression of the VC. Initial linear calcifications were replaced by irregular calcifications (Figures 3B and 4B; mammary glands in 2007).
DISCUSSION
The metabolic mineral and bone disorder associated with CKD (MBD-CKD) is defined as a progressive multifactor systemic disorder, which includes a series of biochemical disorders, bone anomalies and abnormal extra-skeletal calcifications appearing in patients with CKD. Each of these complications results in serious clinical consequences that cause, in turn, increased morbimortality in uraemic patients.9
The presence of VC alters vascular function and structure, which are responsible for the appearance of potentially fatal cardiovascular events like ischaemic heart disease, heart failure, stroke and peripheral vascular disease (PVD). Clinical studies have demonstrated association between the presence of calcification in the arterial intima and/or media layers and a greater risk of cardiovascular and overall mortality.1-6
As a consequence, in recent years, the examination of patients to determine the presence of VC has become an essential part of nephrologists’ daily clinical practice. The aim of KDOQI guidelines and KDIGO proposals is to make an early diagnosis, and individualise treatments to prevent their appearance or reduce their progression, since once established, regression is highly unlikely.7,9-11
Traditional treatments for SPTH and mineral and bone disorders (a diet low in P, calcium-based phosphate binders and vitamin D supplements) are insufficient for most patients and have often resulted in widespread VC. High concentrations of P, Ca and Ca-P product predispose to VC and increase the risk of adverse cardiovascular events.1-6,9-11 Therefore, the treatment should meet the clinical practice guideline criteria as far as possible to minimise adverse effects.
In our patient, use of cinacalcet in combination with vitamin D normalised the Ca, P, Ca-P product and iPTH levels, and managed to keep them within the recommended ranges.
Different clinical studies carried out with cinacalcet have demonstrated its efficacy in achieving proper control of biochemical disorders associated with MBD-CKD, and that the combined treatment of cinacalcet and vitamin D enable reduction of the necessary vitamin D doses thereby reducing its side effects.12-15 In this case, the combination of cinacalcet and intravenous vitamin D treatment enabled correct control of iPTH levels without determining the appearance of hypercalcaemia or hyperphosphoraemia. Hypercalcaemia and hyperphosphoraemia appear when only vitamin D is administered, due to greater intestinal absorption of Ca and P. However, Ca and P serum concentrations are reduced with cinacalcet, as this reduces the flow from the bone in response to PTH reduction, without an additional intestinal mineral source.16 The combined use of both drugs reduces the undesirable effects of vitamin D by mutually counteracting and increasing the inhibition of PTH secretion, thus achieving better control of the biochemical disorders associated with MBD-CKD.
Experimental trials performed on animals have demonstrated that the addition of calcimetics to treatment significatively reduces VC in rats treated with calcitriol and paricalcitol, moreover it may induce regression of extra-osseous calcification. Uraemic rats treated with calcimimetic agents presented a reduction in aortic wall thickness with an increase in calcium receptor expression on the vascular intima and reduction of VC.17-19 This protective effect of cinacalcet against VC has also been published in clinical cases of human patients with CDK and SPTH, as with the case in hand.20,21
Calcium-free binding agents like sevelamer delay development of calcifications and improve their appearance when compared with calcium salts; moreover, they have been associated with a reduction in mortality.22-24 Nevertheless, many studies have shown that the ingestion of calcium salts and subsequent calcium accumulation in the body is associated with VC.1-6,24 In this case both binding agents were used. It is a proven fact that the use of cinacalcet is usually associated with an increase in the calcium carbonate dose.25,26 In this patient, calcium salts were necessary to control hypocalcaemia, initially induced by treatment with cinacalcet. One can see in the initial months that the patient presented a negative calcium balance, which we consider could have been decisive in VC regression in this case. Therefore, the therapeutic treatment used in this patient combining the synergic and antagonistic effects of the drugs used, was highly efficient in controlling SHPT and led to VC regression.
Regression is evident in the mammographies, where we see how linear calcification was replaced by smaller areas of patchy irregular calcification. The images show how clearly VC can be seen in the mammographies, enabling accurate follow-up of their evolution. This is therefore a useful economic and accessible technique for VC diagnosis and follow-up in women with CKD.8,27 The presence of VC in a mammography is considered a cardiovascular risk marker in the general population, which is related to atherosclerosis and diabetes mellitus. However, for the uraemic population, a recently published study establishes the existence of histological correlation between CV presence in a mammography and calcification of the medial layer in arteries.28 Whereas calcification on the arterial intima is essentially related to atheromatosis and inflammation, medial artery calcification has a greater relation to CKD and associated metabolic disorders.5 In this case, given that it is a young woman without additional cardiovascular risks, one might consider that the calcification present in the mammary glands was more related to CKD and mainly affected the medial layer. Were this the case, regression of the mammary calcifications observed could be justified by the correct control of MBD-CKD disorders, since this regression would be unlikely in other patients in whom the VC at the arterial intima and media layers converge due to the interaction of many other factors, thus making the desired regression difficult to achieve.
To conclude, this case has demonstrated that the use of the right treatment can lead to VC regression, and shows the potentially beneficial effect of the calcimimetic in its regression.
Figure 1. (A) Anteroposterior X-ray of left hand in 2006. (B) Anteroposterior X-ray of left hand in 2007
Figure 2. (A) Anteroposterior X-ray of right hand in 2006. (B) Anteroposterior X-ray of right hand in 2007
Figure 3. (A) Anteroposterior X-ray of mammary gland in 2006. (B) Anteroposterior X-ray of mammary gland in 2007
Figure 4. (A) Craniocaudal breast X-ray in 2006. (B) Craniocaudal breast X-ray in 2007
Figure 5. Evolution of Ca, P and serum iPTH levels