Thrombotic microangiopathy (TMA) is characterized by endotelial damage, microangiopathic hemolytic anemia, thrombocytopenia and organ damage, particularly renal. In Oncology, TMA can be secondary to the cancer itself or related to oncological treatments. TMA associated with gemcitabine has a poor prognosis, with high mortality and complement activation plays a central role in its pathophisiology. While eculizumab has shown efficacy and improved outcomes in this condition, evidence regarding the use of ravulizumab remains scarce. We present the case of an 81-year-old woman with arterial hypertension and a history of left breast cancer treated with surgery and radiotherapy, currently diagnosed with stage IV left breast angiosarcoma treated with gemcitabine after progression on paclitaxel. She developed a hypertensive emergency, anemia (Hb 6.9 g/dL), thrombocytopenia (68,000 platelets), impaired renal function (creatinine 1.64 mg/dL) and elevated LDH (1126 U/L). Suspecting gemcitabine-induced TMA, the treatment was discontinued and ravulizumab was initiated, resulting in rapid renal and hematological response. Oncological treatment with pazopanib was reintroduced, leading to recurrence of TMA. That treatment was suspended and another dose of ravulizumab was administered, with good response. TMA is a significant cause of morbidity and mortality in cancer patients, contributing to progression to chronic kidney disease and the discontinuation of oncological treatment. This case highlights the role of ravulizumab in gemcitabine-associated TMA, offering advantages in frail patients due to its longer half-life, reduced administration frequency and favorable outcomes.
La microangiopatía trombótica (MAT) se caracteriza por daño endotelial, anemia hemolítica microangiopática, trombocitopenia y daño orgánico, especialmente renal. En Oncología, la MAT puede ser secundaria al propio cáncer o estar relacionada con los tratamientos oncológicos. La MAT asociada a gemcitabina tiene un pronóstico desfavorable, con alta mortalidad y la activación del complemento tiene un papel central en su fisiopatología. Mientras que eculizumab ha demostrado su eficacia y ha mejorado el pronóstico en esta patología, la evidencia en cuanto al uso de ravulizumab es escasa. Presentamos el caso de una mujer de 81 años con hipertensión arterial, cáncer de mama izquierda tratada con cirugía y radioterapia y actual angiosarcoma de mama izquierda estadío IV tratado con gemcitabina tras progresión con paclitaxel. Desarrolla una emergencia hipertensiva, anemia (Hb 6.9 g/dL), trombocitopenia (68,000 plaquetas), deterioro de la función renal (creatinina 1.64 mg/dL), y aumento de LDH (1126 U/L). Ante la sospecha de MAT inducida por gemcitabina, se suspendió dicho tratamiento y se inició ravulizumab, presentando rápida respuesta renal y hematológica. Se reintrodujo el tratamiento oncológico con pazopanib, con reaparición de la MAT. Se suspendió dicho tratamiento y se administró otra dosis de ravulizumab, con buena respuesta. La MAT es una causa importante de morbimortalidad en los pacientes con cáncer, contribuyendo a la progresión a enfermedad renal crónica y a la suspensión del tratamiento oncológico. Este caso destaca el papel de ravulizumab en la MAT asociada a gemcitabina, ofreciendo ventajas en pacientes frágiles dada su alargada vida media, menor frecuencia de administración y buenos resultados.
Thrombotic microangiopathy (TMA) causes endothelial damage and thrombotic vascular occlusion, resulting in microangiopathic hemolytic anemia, thrombocytopenia, and target organ injury, typically affecting the kidneys and central nervous system.1
In Oncology, TMA can be secondary to the cancer itself or related to cancer treatments.2 Drug-induced TMA has an incidence of more than 15% and is classified into type i (associated with chemotherapy) and type ii (associated with anti-VEGF or tyrosine kinase inhibitor treatments).3 Type I typically occurs during the first 6–12 months of treatment, is dose-dependent and generally is reversible. Its main manifestations are hematological, with high recurrence rates and increased risk of chronic kidney disease (CKD) and mortality. Gemcitabine-associated TMA, being a type I TMA, has an unfavorable prognosis, with renal failure or mortality in 70–75% of cases.4
Complement dysregulation and hyperactivation play a key role in secondary TMA, acting as a "secondary event" that amplifies the endothelial damage triggered by an initial factor and perpetuates TMA. Complement inhibitors such as eculizumab have been effective with secondary TMA.5 Recently, a case series has demonstrated the benefit of eculizumab in the recovery of renal damage in patients with gemcitabine-associated TMA.6
Ravulizumab, a new drug derived from eculizumab, may be an alternative. It offers advantages in terms of its longer half-life (about 52 days vs. ∼11 days), thus allowing longer administration intervals (8 weeks vs. 2 weeks). However, there are currently no studies evaluating the use of ravulizumab in gemcitabine-associated TMA.
Case presentationAn 81-year-old woman presented with a history of arterial hypertension, autoimmune hypothyroidism, left breast cancer treated with surgery and radiotherapy in 2007 and stage iv left breast angiosarcoma with bone involvement diagnosed in December 2021. She started treatment with paclitaxel, switching to gemcitabine after 2 months due to bone progression; she showed a partial response.
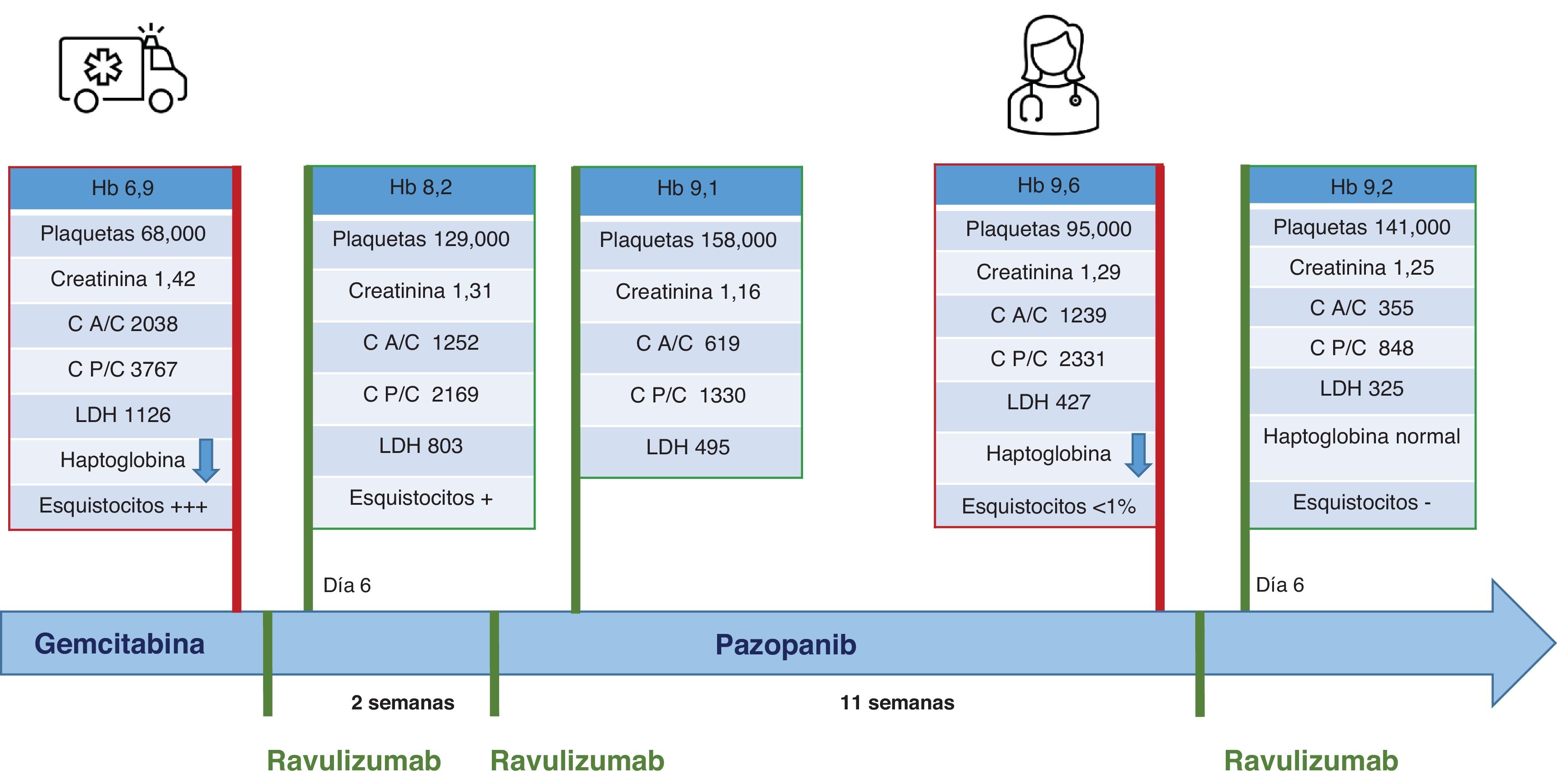
During gemcitabine treatment, she attended the emergency department with a hypertensive emergency (blood pressure 200/110 mmHg), anemia (Hb 6.9 g/dL), thrombocytopenia (68,000/μL platelets), impaired renal function (creatinine 1.42 mg/dL, peak 1.64 mg/dL) and increased LDH (1126 U/L). Urinalysis revealed proteinuria (600 mg/dL on dipstick), an albumin/creatinine ratio of 2038 mg/g, a protein/creatinine ratio of 3767 mg/g, pyuria (>100 leukocytes/field) and microhematuria (5–10 red blood cells/field).
Given the clinical and laboratory findings we suspected gemcitabine-induced TMA. Analysis revealed an undetectable haptoglobin, normal ADAMTS-13 activity and frequent schistocytes in the peripheral blood smear. We considered renal biopsy but could not perform the procedure due to the patient’s functional limitations. Given the clinical suspicion, we discontinued gemcitabine and started ravulizumab based on the patient's frailty, logistical difficulties and our center's experience with that drug. We administered mandatory vaccinations prior to treatment.
Two days after drug administration, both clinical status and laboratory data improved. We discharged the patient on the sixth day, with Hb 8.2 g/dL, 129,000 platelets, creatinine 1.31 mg/dL, LDH 803 U/L, proteinuria 100 mg/dL (by dipstick) and microhematuria (1–5 red cells/field).
A second dose of ravulizumab was administered at 13 days, as recommended, and renal function remained stable (creatinine 1.3 mg/dL, eGFR 40 mL/min), Hb 9.1 g/dL, 158,000 platelets, LDH 495 U/L.
One month after the last drug administration, the oncologists decided to restart oncologic treatment with pazopanib. After 9 weeks of treatment, anemia reappeared (Hb 9.6 g/dl), with decreased haptoglobin and moderate elevation of LDH (427 U/L). Tyrosine kinase inhibitors are known to be associated with type ii TMA, so, suspecting a recurrence of TMA, we discontinued pazopanib and administered another dose of ravulizumab. Renal function remained stable (creatinine 1.2–1.4 mg/dL), with no signs of hemolysis (Fig. 1). We started cyclophosphamide, after which the patient remained stable for the next 5 months.
Discussion and conclusionsStage 3–5 CKD affects 12–25% of cancer patients7 and significantly increases cancer-related mortality as well as all-cause mortality. Oncological treatments are an important cause of nephrotoxicity, so we highlight the importance of understanding the mechanisms of renal damage and implementing multidisciplinary management.
A Spanish study showed that TMA is the fifth most frequent diagnosis in cancer patients undergoing renal biopsy8. Given the significant morbidity and mortality of TMA, diagnosing and treating it early is vital to reduce mortality and CKD progression. In addition, recovering renal function is crucial for continuing oncological treatment, which ultimately determines the prognosis of patients. Therefore, whenever possible, clinicians should avoid oncological drugs known to cause TMA.
In our case, we highlight the benefits of ravulizumab in a patient with gemcitabine-induced TMA, who presented a recurrence after administration of pazopanib. Ravulizumab is especially beneficial in frail and mobility-limited patients because of its therapeutic schedule, offering both clinical benefits and improvements in quality-of-life.
Although the evidence for the use of ravulizumab in TMA associated with chemotherapy is scarce and limited to a single published clinical case in a young patient with pancreatic cancer9, the case presented here provides further evidence that ravulizumab may be a practical and effective option for these patients.
FundingThe present research did not receive funding from public sector agencies, private companies or non-profit entities.
Clara García Carro has received financial support for attendance at congresses and scientific meetings from Alexion and has received payment for collaborations in the form of lectures and writing scientific material for Alexion.
The authors of this manuscript declare that they have no conflicts of interest.