Uremic pruritus (UP) is one of the most uncomfortable symptoms for patients in dialysis. UP has a great impact on dialysis patients’ quality of life and has a great prevalence between those (28–70%). Physiopathology of UP is unknown and usually is unnoticed for most nephrologists (in more than 65% of centers is underdiagnosed). This lack of awareness drives to the unsuccessful treatment of this symptom. Moreover, the fact that most studies have been carried out on small populations and the difficulty assessing UP complicates a correct therapeutical approach. For this reason, we have designed treatment algorithms based on the efficacy of the drugs but also its safeness to avoid adverse effects.
El prurito es uno de los síntomas más incómodos y que más impacta en la calidad de vida de los pacientes en diálisis. Su prevalencia es bastante elevada en pacientes en diálisis (28-70%). La fisiopatología del prurito urémico es desconocida, y este síntoma a menudo pasa desapercibido para el personal sanitario, siendo infradiagnosticado en más del 65% de los centros. Esta falta de reconocimiento deriva en un abordaje terapéutico ineficaz del prurito urémico. Por otro lado, la mayoría de los ensayos farmacológicos para el tratamiento del prurito urémico han sido realizados en poblaciones reducidas y están sujetos a la subjetiva medición del propio síntoma. Por este motivo, hemos propuesto algoritmos de tratamiento, teniendo en cuenta la evidencia que avala a cada fármaco y a la vez la pluripatología y la polifarmacia de cada paciente, con el fin de evitar efectos adversos.
Among the symptoms associated with chronic kidney disease (CKD), pruritus stands out,1 and it is particularly frequent in patients on regular dialysis. Its prevalence varies according to the series reported and technique of the renal replacement therapy being used. One study that compared the frequency and intensity of pruritus in hemodialysis (HD) and peritoneal dialysis (PD) patients, it was observed that 38.2% of HD patients presented pruritus, compared to 28.6% of patients on PD, being also more intense in the HD patients.2 However other studies show that the prevalence of itching is 12-15% higher and more intense in PD patients.3,4 Two studies hypothesize that these results may be due to the greater clearance of medium-sized molecules, such as β2-microglobulin, with modern HD than with PD. Some studies estimate the prevalence of pruritus in dialysis patients at around 70%, without distinguishing between techniques.5 However, it could be that there is lower prevalence of pruritus in recent years. The DOPPS study reported that, from 1996 to 2015, the percentage of patients suffering from intense or extremely intense pruritus has decreased from 28% to 18%.6
However, despite the fact that the prevalence of pruritus remains high, it is a symptom that often goes unnoticed in dialysis centers. It is estimated that 65% of HD unit managers believe they have a prevalence of pruritus of less than 5% among their patients, but the reality it ranges between 21-50%. Moreover, up to 17% of patients with pruritus did not report this symptom to health personnel. Likewise, the centers with the lowest prevalence of pruritus were those that best estimated their own prevalence, and this could mean that a greater awareness of the problem would lead to a better recognition and approach to the pruritus.7
Despite the importance, discomfort and impact on the quality of life caused by pruritus, there is little information on its treatment, and there is much variability in the therapeutic approach. Now days when doctors are more aware of a patient-centered medicine, we consider necessary to carry out a review on pruritus in dialysis patients.
What is itching and how is it measured?Pruritus is the perception of itching in one part of the body or all over it that causes the need to scratch. The evaluation of its intensity is complex as it is a fairly subjective symptom; and the most used tool to evaluate it is the visual analog scale of pruritus. This scale goes from 0 to 10, where 0 is the absence of itching and 10 is the most itching imaginable.
Although the visual analog scale is the most used tool, it is a one-dimensional scale, since it only evaluates the intensity of itching. Other scales also assess the distribution of pruritus and its impact on sleep; this would be the case of the modified Pauli-Magnus scale.3 The 5-D itching scale also evaluates the effect of itching on quality of life.8 Lastly, the Skindex-10 adds to the previous ones the emotional, social and occupational impact of pruritus in the lives of patients.7
Pathophysiology of pruritus in chronic kidney diseaseThere is considerable uncertainty regarding the pathophysiology of pruritus in CKD. Numerous theories have been developed, and it has not been possible to prove a single origin. It is thought that multiple factors may contribute to its development.
Pruritus has traditionally been associated with alterations in calcium/phosphorus metabolism. The association between hyperparathyroidism and pruritus has been described, the latter improving after parathyroidectomy.9,10 In addition, high plasma phosphorus levels (> 5.5 mg/dl) and elevated calcium-phosphorus product have also been associated with its development.6 In mice, CaP injection was shown to induce pruritus mediated by the release of IL-6.11
Malnutrition is another factor that has been linked to itching. The presence of analytical parameters compatible with malnutrition, such as low albumin levels and elevated C-reactive protein, are more frequent among patients with pruritus.12,13 But there is no good explanation to link these phenomena with itching.
It has been observed that an alteration of primary afferent sensory neurons or interneurons may favor the appearance of pruritus14; and there is a high prevalence of neuropathy and dysautonomia in dialysis patients, and a higher prevalence of these neurological deficits in patients with pruritus.15 This could explain the high prevalence of pruritus in dialysis patients and could justify the good response of pruritus to drugs used to treat neuropathic pain.
In relation to the state of cutaneous hydration and the presence of itching, it is known the continuous and sudden changes in the hydration state of HD patients, leading in many cases to states of dehydration that favor the appearance of xerosis, another factor related to the appearance or worsening of pruritus.7,16 It has been observed in the skin of patients with CKD there is greater atrophy of the sebaceous glands, a decrease in sweat glands and there is a lower water content in the stratum corneum.17 Interestingly, it seems that xerosis is more common in PD patients.4
The adequacy of dialysis has also been related to itching. Low-flow dialysis and urea Kt/V levels below 1.5 have been associated with worse evolution and worsening over time.18 In the DOPPS-I study, differences were also found in the prevalence of pruritus based on Kt/V, but this has not been observed in the DOPPS-II.6
Finally, perhaps the most important hypothesis to explain the generation of pruritus is that of deregulation of the opioid system, since it is the main target of many of the drugs used in the treatment of uremic pruritus. It is hypothesized that in CKD patients there is an imbalance between µ and κ opioid receptors, which are mutually antagonistic,19 with a disparity in favor of µ receptors. Although the exact mechanism is unknown, pruritus is a common adverse effect after the administration of µ agonists, and could be mediated by modulation of serotonergic transmission, activation of the dorsal horn and the itch center in the central nervous system.20 Wieczorek et al. skin biopsies were performed on 40 HD patients, of whom 21 had itching and 19 did not; and evaluated the amount of µ and κ receptors in the skin of the 2 groups of patients, finding significant differences in the expression of κ receptors (lower in patients with pruritus, showing an inversely proportional relationship with its intensity). However, they did not observe differences in the expression of µ receptors between patients with and without pruritus.21
Body distribution and characteristics of uremic pruritusUremic pruritus has a heterogeneous distribution, in some cases it is a generalized,22 while in other cases it can be located on the back, face or arms.23 It is usually symmetrical, and in terms of its frequency of appearance it can be daily, or in up to 25% of cases it occurs at the end of the dialysis session.19
Quality of life and mortalityPruritus, whether moderate or severe, can be very limiting and may affect significantly the quality of life of patients. It can alter physical capacity, make it difficult to carry out the basic tasks of daily life, favor the appearance of sleep disorders and reduce the ability to enjoy social relationships. However, it does not seem to affect the sexual life of patients.24
The itching may not be as trivial as it may initially seem. Some studies have shown that the presence of severe pruritus is associated with a worse prognosis and higher mortality.22 In the DOPPS study, mortality was found to be 17% higher in patients with moderate to extreme pruritus. However, after adjusting for sleep quality, the association between pruritus and mortality disappeared.6
Pharmacological and non-pharmacological therapeutic approachesTopical treatmentsThey constitute one of the fundamental treatments for uremic pruritus, since dialysis patients often present with xerosis due to the presence of alterations in the cutaneous vascularization and the decrease in sebaceous and sweat glands that favor dry skin.16 For this reason, topical treatments, and especially emollients, are one of the main therapeutic options when it comes to rehydrating the skin in a population with a high prevalence of xerosis at baseline.19 Tacrolimus ointments have been shown to be effective in a small cohort of patients with a reduction in pruritus of up to 80%.25 However, the effectiveness of this treatment is lost within a few weeks of discontinuation of the treatment.
Dialysis filtersPolymethyl methacrylate (PMMA) filters could have some influence in the treatment of itching through their adsorption capacity of medium and high molecular weight particles. In patients dialyzed with PMMA membranes, there is a decreases of 1-2 points in the visual analog scale of pruritus.26 Another possible mechanism would be the decrease in TNF-α; however, in one study, PMMA dialyzers were shown to reduce pruritus independently of TNF-α concentrations.27
PhototherapyUltraviolet B (UVB) radiation could help improve uraemic pruritus, although the latest published clinical trial did not show benefits compared to the control group.28 On the other hand, these patients have a greater predisposition to skin cancer due to their immunosuppression situation, in the past (previous transplant recipients), current (due to kidney disease itself) or future (future transplants). It is necessary to weigh the risks and benefits of this treatment.
Gabapentin and PregabalinBoth drugs are anticonvulsants and act by blocking calcium channels centrally. In addition, they modulate the neuropathic pain, which is why they have been used in uremic pruritus. In a double-blind clinical trial of 25 patients, gabapentin at a dose of 300 mg/3 times a week was shown to reduce pruritus on the visual analog scale up to 7 points without major adverse effects, although it should be noted that the duration of treatment it was only 4 weeks.29 In another study, reductions of more than 7 points were achieved by administration of less doses of gabapentin post-dialysis (100 mg, 3 times per week).30 However, in some series up to 37% of patients discontinued the treatment due to adverse effects. In these cases, switching to pregabalin (25 mg daily in PD patients and 25 mg post-HD in HD patients) can achieve comparable effects with better tolerance.31
A systematic review on the treatment of pruritus concludes that the only drug with a proven effect on uremic pruritus would be gabapentin.32
Sodium cromoglycateIts possible effect in reducing pruritus has been based on stabilizing mast cells with the reduction of tryptase. Surprisingly, in a trial carried out with the drug, pruritus was significantly reduced, although the basal levels of serum tryptase were not reduced (dose of 135 mg 4 times a week).33
DifelikefalinIt is the newest drug for the treatment of pruritus. It exerts its effect by its selective agonistic action on the K receptors of peripheral neurons. In a clinical trial (phase 3) in which it was administered intravenously during HD sessions, it was shown to be effective since the first week. 51.9% of the patients treated with difelikefalin achieved a reduction in pruritus of at least 3 points on the visual analog scale for itching, while only 30.9% in the placebo arm achieved this reduction of pruritus.34 In addition, it improved the quality of life due to reduction of pruritus. The most frequently reported adverse effects were diarrhoea, vomiting and dizziness.
MontelukastUsed especially in childhood asthma, it has anti-inflammatory action through the inhibition of leukotrienes. The study with montelukast in uremic pruritus at daily doses of 10 mg achieved a decrease of 3.7 points in the visual analog scale of pruritus of.35 It was also shown to significantly reduce C-reactive protein levels, so its effects could be due to its anti-inflammatory action. It is noteworthy that it is a very safe drug with few adverse effects and few interactions with other medications.
MirtazapineIt is a serotonergic antagonist of 5HT2 and 5HT3 receptors, and also has antihistaminic action by acting on H1 receptors. The evidence of a positive effect on uremic pruritus comes from a study in 77 patients who were treated in a sequential trial.36 In this trial, gabapentin 100 mg per day was administered for 2 weeks, followed by a 2-week washout period, and finally patients received mirtazapine 15 mg per day. A decrease in the visual analog scale of pruritus of 5.3 points was obtained with mirtazapine and 3.96 with gabapentin. In addition, 62% of patients preferred treatment with mirtazapine, despite having more adverse effects.
SertralineIts use is based on its efficacy in treating pruritus of cholestatic origin. The mechanism by which it could reduce uremic pruritus is unknown, although it has been shown to be effective at a dose of 50 mg per day,37 with the advantage that it does not need to be adjusted to renal function.
AntihistaminesThese drugs, widely used in immunoallergic processes and in pruritus of other aetiologies, have not been shown to be effective in the treatment of uremic pruritus in patients with CKD.22,38
NaltrexoneIts action is mediated by being an antagonist of the µ receptor. In a double-blind, crossover trial that included 15 HD patients with extreme refractory pruritus (mean 9.9 out of 10 on the visual analog scale for pruritus), it was shown that doses of 50 mg per day of naltrexone were effective in eliminating pruritus almost completely.39 However, a later study with a slightly larger cohort of patients and of longer duration failed to show benefits and there was a high percentage of adverse effects.40
NalfurafineIt is a highly selective drug for κ opioid receptors, widely used in Japan and not marketed in Spain. In a clinical trial of 337 dialysis patients, it was shown to reduce itching compared to placebo by an average of 2.2 on the visual analogue scale of itching. However, it produced insomnia in up to 15% of patients taking the highest dose.41
OndansetronThere are only 2 trials in which ondansetron was compared to placebo, and both with a very small cohort. The justification for its use was that ondansetron antagonizes the serotonergic action involved in the transmission of pain and pruritus; however, both studies did not show a benefit as compared to placebo.42,43
DiscussionPruritus is one of the most bothersome symptoms for dialysis patients, its appearance is quite frequent in this population (around 40%).2–4,6 There are discrepancies regarding the prevalence of pruritus in the different dialysis modalities; some studies conclude that it is more common in HD patients2 and others show the opposite.3,4 Despite its high prevalence and its great impact on the quality of life of patients, it is often underdiagnosed and, therefore, undertreated in many centers.7
The etiology of dialysis pruritus is not clearly defined. Multiple factors have been related to its appearance, which would explain why it is a symptom that is difficult to control and with a variable response to the different drugs used.
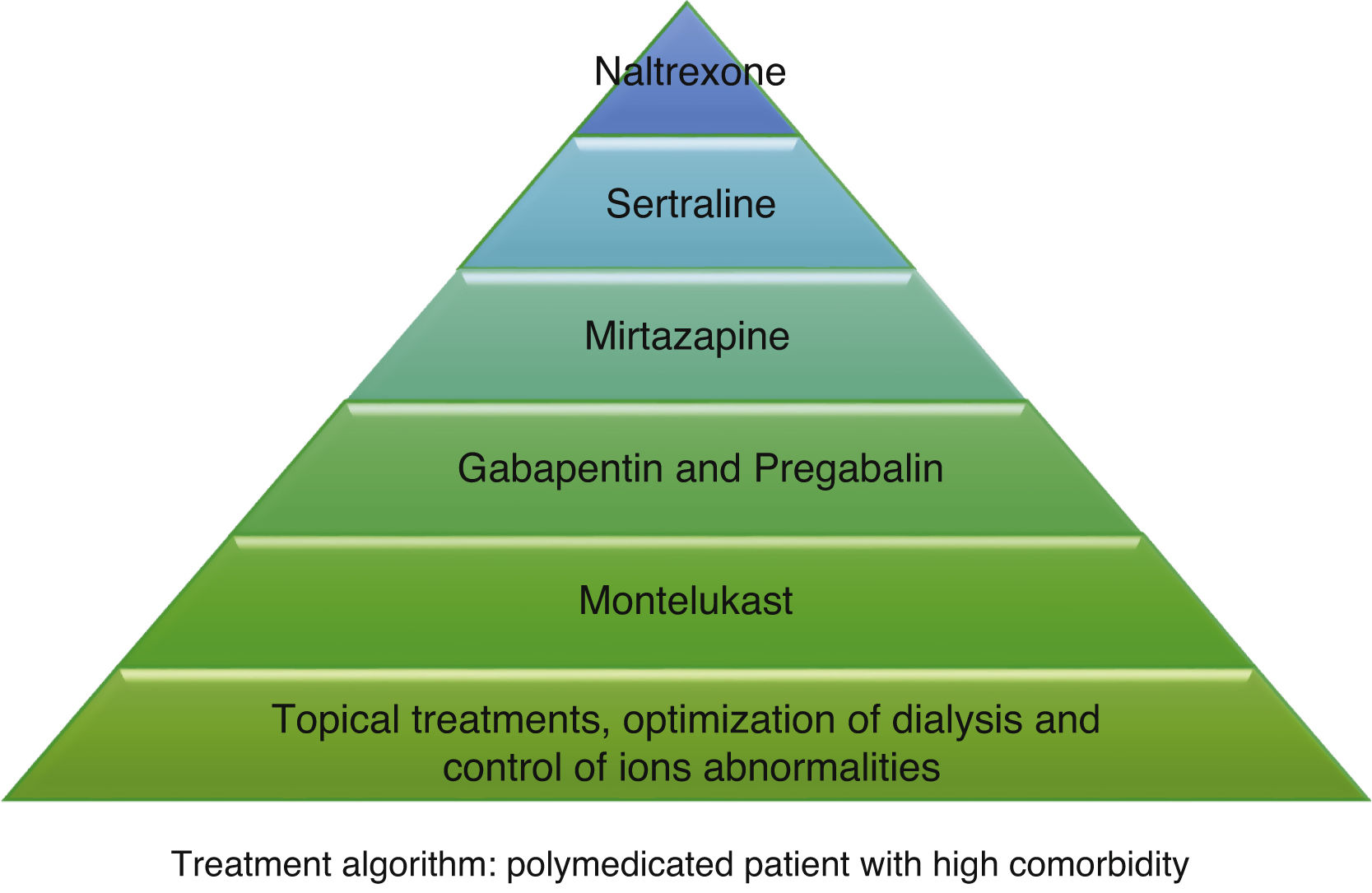
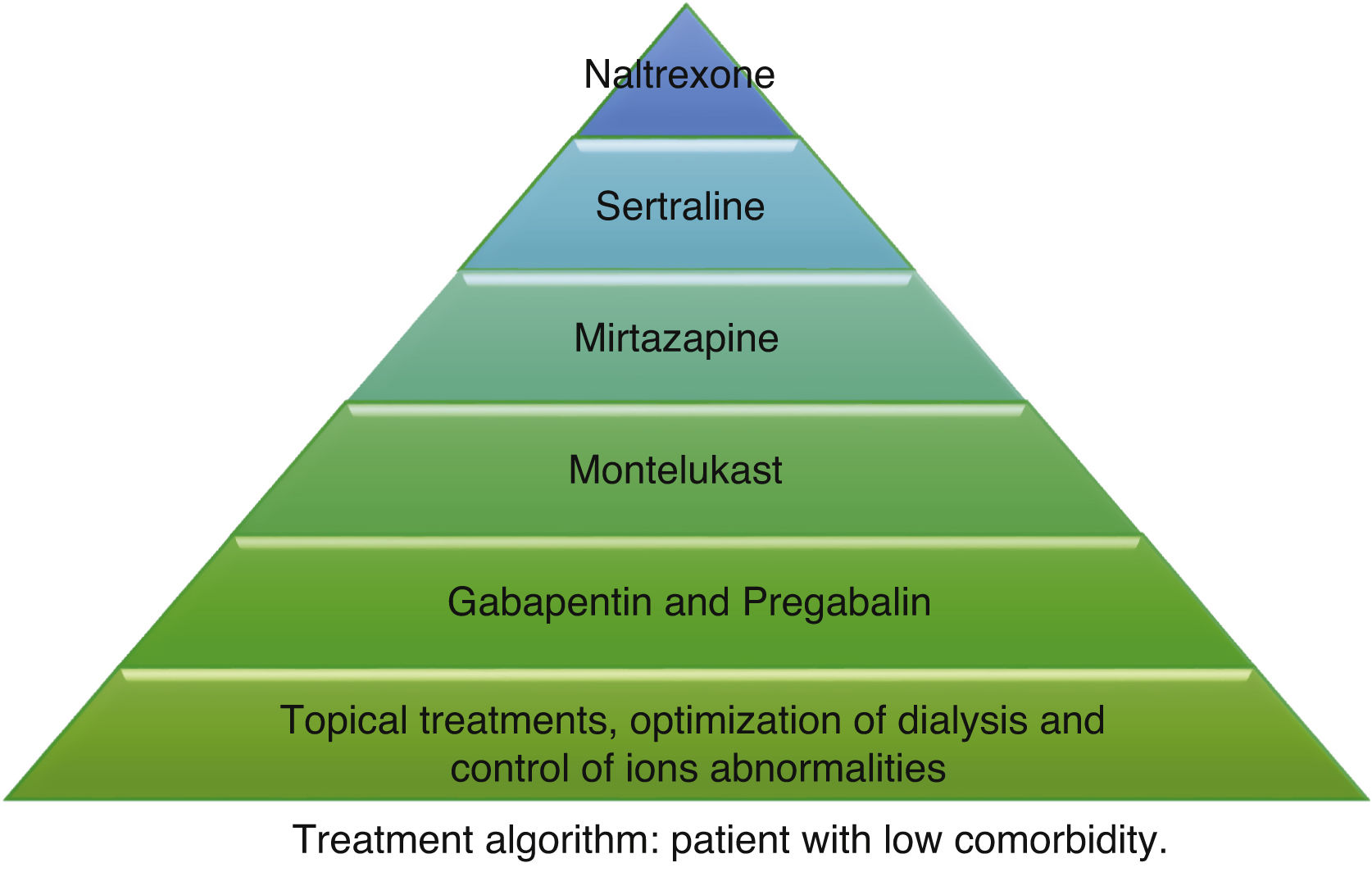
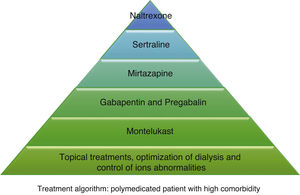
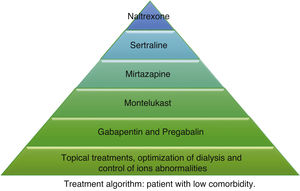
According to the information collected, we have developed 2 different treatment algorithms based on the baseline situation and comorbidities of the patients. Fig. 1 shows what should be the approach of the treatment of pruritus in elderly patients with many comorbidities, and Fig. 2 deals with patients with better baseline status and less frail. When designing the algorithm in Fig. 1, we have prioritized those treatments that have most evidence and the best safety profile. Since most of the existing clinical trials are small and of short duration, their evidence could be considered limited. For this reason, and given that many of the dialysis patients are frail and polymedicated, we consider a priority to treat pruritus with safe drugs and with the fewest drug interactions possible in these patients. The first step in both algorithms is to be most conservative, and would consist of adapting dialysis and controlling the calcium-phosphorus product as much as possible, added to the application of topical treatment with emollients. The second step in the algorithm in Fig. 1 would consist of taking 10 mg of montelukast daily at dinner due to its proven efficacy and, above all, due to its tolerance.35 The third step of the algorithm in Fig. 1 would be gabapentin, starting at a dose of 100 mg post-dialysis, with the possibility of escalating the dose later if the expected effect is not achieved. This is the drug with the most evidence of being effective in the treatment of pruritus in dialysis,29,30,32 which is why it is our second step in the algorithm in Fig. 2. However, in comorbid patients we prefer to relegate gabapentin to a third step and try first with other safer options. In cases where gabapentin is poorly tolerated, pregabalin may be an equally effective and better tolerated drug.31 The fourth step in both algorithms would be mirtazapine at a dose of 15 mg daily. In a single trial, it demonstrated more efficacy than gabapentin, however, as it was only one study and presented more adverse effects, we considered it the fourth option. Sertraline at a dose of 50 mg daily would occupy the 5th position. Finally, in those patients with very poor control, the use of naltrexone could be considered.
Other drugs such as nalfurafine and sodium cromoglycate, despite their efficacy,33,41 are not included in our algorithm since we do not have access to these drugs in our center, but they could be options to be assessed if they are available. Phototherapy has been dismissed due to the predisposition of these patients to skin cancer, considering the risk greater than the potential benefit. Difelikefalin appears to be quite safe and shows promising results,34 so it could be considered when it becomes marketed.
Finally, despite the frequency with which antihistamines are used in the treatment of uremic pruritus, being the most used drug in many centers (up to 57% used it as first choice),7 there is no evidence on its efficacy.
In conclusion, uremic pruritus is an underdiagnosed symptom, and with a poor therapeutic approach in many cases. Probably, with a directed and systematized treatment, a better control could be achieved with improvement in the quality of life of patients.
Conflict of interestsThe authors declare that they have no potential conflict of interest related to the contents of this article.
Please cite this article as: Santos-Alonso C, Maldonado Martín M, Sánchez Villanueva R, Álvarez García L, Vaca Gallardo MA, Bajo Rubio MA, et al. Prurito en pacientes en diálisis. Revisión de la literatura y nuevas perspectivas. Nefrologia. 2022;42:15–21.