Antecedentes y objetivos: Los pacientes en hemodiálisis tienen un riesgo aumentado de padecer infecciones en comparación con sujetos no en diálisis. La procalcitonina se eleva en pacientes con infecciones bacterianas. Sin embargo, no ha sido estudiada en los pacientes en diálisis. El objetivo del presente estudio es evaluar la procalcitonina como predictor precoz de infecciones en los pacientes en hemodiálisis. Métodos: Se trata de un estudio de cohortes retrospectivo con 211 pacientes prevalentes en hemodiálisis (mediana de edad: 73 años [rango 60-80], 58 % varones) entre 2005-2012. Se extrajeron muestras prediálisis y se siguió a los pacientes durante 40 ± 25 meses (0-84). Se recogieron datos basales demográficos y de laboratorio, incluyendo marcadores inflamatorios. Durante el seguimiento se documentaron y analizaron las nuevas infecciones. Resultados: Durante el seguimiento, 112 pacientes (53 %) tuvieron una infección. Se estableció una correlación entre procalcitonina y proteína C reactiva (PCR) (σ = 0,482, p < 0,0001). El único marcador estudiado capaz de predecir infecciones al primer mes fue la procalcitonina (p = 0,023) en un modelo ajustado. La PCR fue el mejor predictor de infección durante el seguimiento global (p = 0,003), en un modelo ajustado. Conclusiones: La procalcitonina es un marcador de infección precoz (a 30 días) en los pacientes en hemodiálisis. Sin embargo, la PCR resultó ser el único marcador asociado con infecciones a largo plazo.
Background and aims: Hemodialysis patients have a greater risk of infection than individuals not on dialysis. Procalcitonin has been shown to rise in bacterial from but widely studied in hemodialysis patients. The present study evaluates procalcitonin as an early predictor of infection in this population. Methods: A historical cohorts study was made of 211 prevalent hemodialysis patients (median age 73 years [range 60-80], 58% males) covering the period 2005-2012. Serum samples were thawed and patients were followed-up on for 40±25 months (0-84). Demographic and laboratory test (including inflammatory values) data were recorded at baseline. During follow-up, all infections were documented and analyzed. Results: During follow-up, 112 patients (53.3%) suffered acute infection. A positive correlation was established for procalcitonin and C-reactive protein (σ=0.482, p<0.0001). Procalcitonin was the only inflammatory marker capable of predicting infection at one month (p=0.023) in a model with all the studied inflammatory markers. C-reactive protein was the best predictor of infection over global follow-up (p=0.003), after adjusting for all the studied factors. Conclusions: Procalcitonin is an early predictor of infection in the first 30 days in hemodialysis patients. However, in relation to the long-term prognosis, C-reactive protein is the most important independent predictor of infection.
INTRODUCTION
Inflammation is an established mortality risk factor in chronic kidney disease patients and particularly in patients on dialysis.1 Individuals with a decreased glomerular filtration rate have elevated levels of inflammatory markers that increase with the extent of renal damage and are caused by oxidative stress, endothelial dysfunction, and vascular calcification.2,3 Thus, since inflammation is a cardiovascular risk marker in hemodialysis (HD), finding the best predictor of inflammation is a priority concern for nephrologists.4 Many biomarkers have been evaluated in the last 20 years, though no clear consensus has been reached.5,6
On the other hand, infection is a major cause of morbidity and mortality in the population on dialysis.7 The clinical manifestations of infection in such patients differ from those of individuals not on dialysis; inflammatory biomarkers are therefore useful for evaluating the presence of infection. The difficulty involved is that the classical inflammatory markers are elevated at baseline in patients on dialysis.8
The objective of the present study was to assess the capacity of procalcitonin to detect acute infections in hemodialysis patients compared with other known inflammatory markers such as albumin or C-reactive protein (CRP).
MATERIALS AND METHODS
A total of 245 HD patients from a single center were enrolled and followed in this historical cohort retrospective study. The inclusion criteria were: stable patients with no recent infections and hospitalizations in the four weeks before serum determinations (including surgery). Patients with criteria of MIA syndrome (malnutrition-inflammation-atherosclerosis) were excluded. Of them, 211 met inclusion criteria and were finally enrolled. All patients had four hours HD treatment, three times per week, and high-flux polysulfone filters. The mean of Kt/V was 1.7±0.3.
Baseline characteristics were recorded, including age, sex, time on hemodialysis and cardiovascular risk factors (diabetes mellitus, previous dyslipidemia,9 previous hypertension10 and a history of cardiovascular disease). C-reactive protein (CRP) (measured by immunofluorescent assay), procalcitonin (LUMItest®-procalcitonin-kit, B.R.A.H.M.S.-Diagnostica, Berlin, Germany), white blood cell count, and albumin were measured at baseline. Serum pre-dialysis samples were collected between 2005 and 2007 and stored at -30°C. In December 2011, all the samples were thawed and analyzed at the same time. Two groups were established for each of the parameters (CRP, procalcitonin and albumin), in order to determine their predictive value referred to infection after the first and fourth month, and during global follow-up, above and below the median. Correlations between these biomarkers were established.
Routine clinical and biochemical variables were measured by standardized methods on autoanalyzers.
New infectious events11 were recorded during follow-up (median months 39 [range 19-56]), and were codified as respiratory, vascular access, skin, gastrointestinal, urinary and other infections. Sepsis was defined when an infection was suspected or confirmed by cultures and meet two of the following criteria: body temperature more than 38ºC or less than 36ºC, heart rate upper than 90 beat per minute, respiratory rate over than 20 per minute, white blood cell count over than 12,000/µL or lower than 4,000/µL. Infections must met sepsis criteria for been included. All of them required hospitalization. All of them required hospitalization and results of the cultures were recorded. An analysis was made of the factors independently predicting such events.
Values are expressed as the mean (SD) or median (IQR). Univariate and multivariate analyses were performed by Cox regression. Analysis of variance was used when several parameters of the two groups were compared. Correlations were established using the Pearson test. Variables with p<0.1 in the univariate analysis and those regarded as confounders were entered in the multivariate analysis. Receiver operating characteristic (ROC) curves were drawn to establish the sensitivity and specificity points in order to find a cut-off value for biomarkers. All statistical analyses were performed with SPSS® 18.0 (Chicago, IL, USA). Statistical significance was considered for p<0.05.
RESULTS
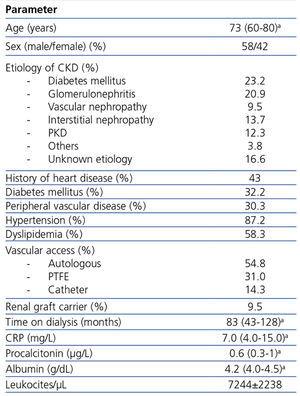
The baseline characteristics are shown in Table 1.
Study of infection
During follow-up, 112 patients (53.3%) suffered infection (Figure 1). Of these subjects, 12 developed infection in the first month, and 22 in the first four months. CRP showed a positive correlation to procalcitonin (σ 0.482, p<0.0001) and a negative correlation to albumin (σ -0.256, p=0.002). No association was found between procalcitonin and albumin; and between leukocytes and any biomarker. Also, no differences were found between diabetic and non-diabetic patients in procalcitonin and CRP levels. Patients had positive cultures in 26% of the cases. No differences were found between levels of procalcitonin and positive and negative cultures (p=0.07), but when analyzing only vascular access infections (n=28, 25%), patients with positive cultures (50%) had higher levels of procalcitonin when compared to those with negative ones (1.38 vs 0.50µg/L, p=0.043).
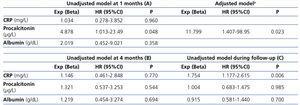
The infection predicting capacity of the inflammatory parameters is shown in Table 2 A. Procalcitonin was found to be the only independent predictive factor for infection at the first month in the univariate analysis, along with age and gender following adjustment for the rest of the parameters. No relation was found between procalcitonin and etiology of CKD (p=0.34). The ROC values were 0.636 (95%CI 0.469-0.802, p=0.126) and 0.506 (95%CI 0.315-0.696, p=0.946) for procalcitonin and CRP, respectively. Cut-off values for procalcitonin for 1-month infection resulted in 0.65µg/L.
The same analysis was performed to determine the infection predicting capacity of the different biomarkers at the fourth month. As shown in Table 2 B, none of them were associated to the end variable.
Lastly, the univariate analysis revealed an association between CRP and infection during follow-up (Table 2 C).
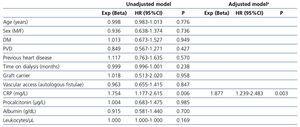
A final analysis was made (Table 3) including all the collected factors in order to determine the predictors of infection in patients on hemodialysis. The univariate and multivariate analyses identified CRP as the only long-term predictor of infection. Cut-off values for it for infection resulted in 1.9mg/L and for inflammation 1.1mg/dL. For assessing the procalcitonin capacity for predicting infectious events irrespective of the inflammation status of the patient, we compared their levels in patients to CRP levels over and below the cut-off for inflammation and no differences were found (0.6 vs 1.2µg/L, p=0.217).
Vascular access study
We analyzed the association between procalcitonin and CRP and the dialysis vascular access. No significant differences were found between procalcitonin levels in patients with polytetrafluoroethylene (PTFE) and catheter (median 0.600 [0.400-0.925]) and an autologous fistula (median 0.550 [0.275-1.100]) (p=0.303). No independent predictor was found in the multivariate models for elevating procalcitonin. Likewise, procalcitonin in patients with vascular access infection did not differ from that in patients with other infections (p=0.629).
No significant association was found between CRP levels in patients with PTFE and catheter (median 0.800 [0.400-1.825]) and an autologous fistula (median 0.600 [0.300-1.425]) (p=0.070). Linear regression analysis adjusted for a history of heart disease, renal graft carrier, gender, age and cardiovascular risk factors, identified time on dialysis (B 0.007 [0.002-0.012] p=0.007) and vascular access other than autologous fistulae (B 1.034 [0.154-1.914] p=0.022) as the only independent predictors of CRP elevation. In the same way as procalcitonin, CRP showed no differences in concentration between patients with vascular access infections and other infections (p=0.849).
DISCUSSION
Our study confirms the importance of procalcitonin as a diagnostic biomarker when infection is suspected in patients on hemodialysis. To our knowledge, the present study comprises the largest sample of procalcitonin measurements in HD patients published to date.
Classical acute phase reactants (e.g., CRP, interleukins, albumin, fibrinogen, etc.) are increased in chronic kidney disease, and particularly in chronic dialysis patients, due to their inflammatory status. Several additional factors contribute to increased levels of inflammatory markers, namely periodontitis, the retention of circulating cytokines, non-biocompatible hemodialysis membranes, volume overload, and subclinical infections of the vascular access routes.2,12 Such asymptomatic elevations are associated with increased cardiovascular morbidity-mortality, and it is difficult to interpret their values in these patients.13,14
Procalcitonin, a 116-amino acid precursor protein of calcitonin, has been shown to be able to accurately distinguish bacterial from nonbacterial infections.15 It has not been widely studied in populations with chronic kidney disease, and only one small study included hemodialysis patients.16 Herget-Rosenthal etal. studied 68 hospitalized patients receiving hemodialysis. They prospectively followed-up on the patients, finding that procalcitonin was associated with the development of sepsis (n=12) in comparison with patients without infection (p<0.01). In coincidence with our own results, they found procalcitonin to be more a powerful predictor of acute infection than CRP.15 In a recent metaanalysis of peritoneal dialysis, hemodialysis and chronic kidney disease, procalcitonin showed the same usefulness as CRP in diagnosing infection, but the analysis only included one study in hemodialysis patients.17 As expected, procalcitonin concentration did not increase when infection occurred beyond the fourth month.
Infections in hemodialysis patients have more impact in morbidity and mortality when compared with general population. The possibility of detecting subclinical infection could let us early treatments. The most difficult infection to identify is probably vascular access infection, due to its atypical presentation. Periodically monitored procalcitonin levels can give us the possibility of detecting subclinical infections, including asymptomatic vascular access infections. Our study cannot confirm this hypothesis, but basal levels of procalcitonin in asymptomatic patients were higher in positive culture infections of the vascular access, showing its important role in subclinical infections.
CRP levels were higher in patients with vascular accesses other than autologous fistulae. Also, vascular access and time on dialysis were independent predictors of the elevation of this biomarker, showing them to make a contribution in the microinflammatory environment of dialysis patients. This situation was previously reported by Goldstein et al., who described a reduction in CRP when catheters were substituted by fistulae in the hemodialysis population.17 More recently, Colì et al. recorded increased inflammation (including CRP) in patients without an autologous fistula when compared with PTFE and catheters.18 We found no association between procalcitonin and vascular access.
Several studies have demonstrated a strong association between low albumin levels, cardiovascular events and mortality in dialysis patients.19,20 However, no association was found between acute infection and albumin in our study.
The present study has some limitations that must be commented. Firstly, it is a non-randomized study, and has the typical limitations of its retrospective design. Secondly, the diagnosis of infection was performed by different specialists. To avoid this bias, we followed standardized criteria in defining new infections.11 Also, we only had a sample were inflammatory biomarkers were measured, and monitoring patients with more than one could give us information about the variation of its levels and the effect of the dialysis.
CONCLUSIONS
Procalcitonin is the best of the studied biomarkers for acute infection in hemodialysis patients. CRP reflects chronic inflammation, is related to vascular access, and can be useful as a stratifying tool for detecting patients at high risk for long-term infections.
Conflict of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Baseline characteristics
Figure 1. Infectious events developed during follow-up.
Table 2. Univariate and Cox regression analysis for inflammatory markers (above and below their respective medians) predicting infection at the first month (A). Unadjusted models predicting infection at the fourth month (B) and during global follow-up (C)
Table 3. Univariate and Cox regression analysis for factors predicting infections during follow-up