To the Editor
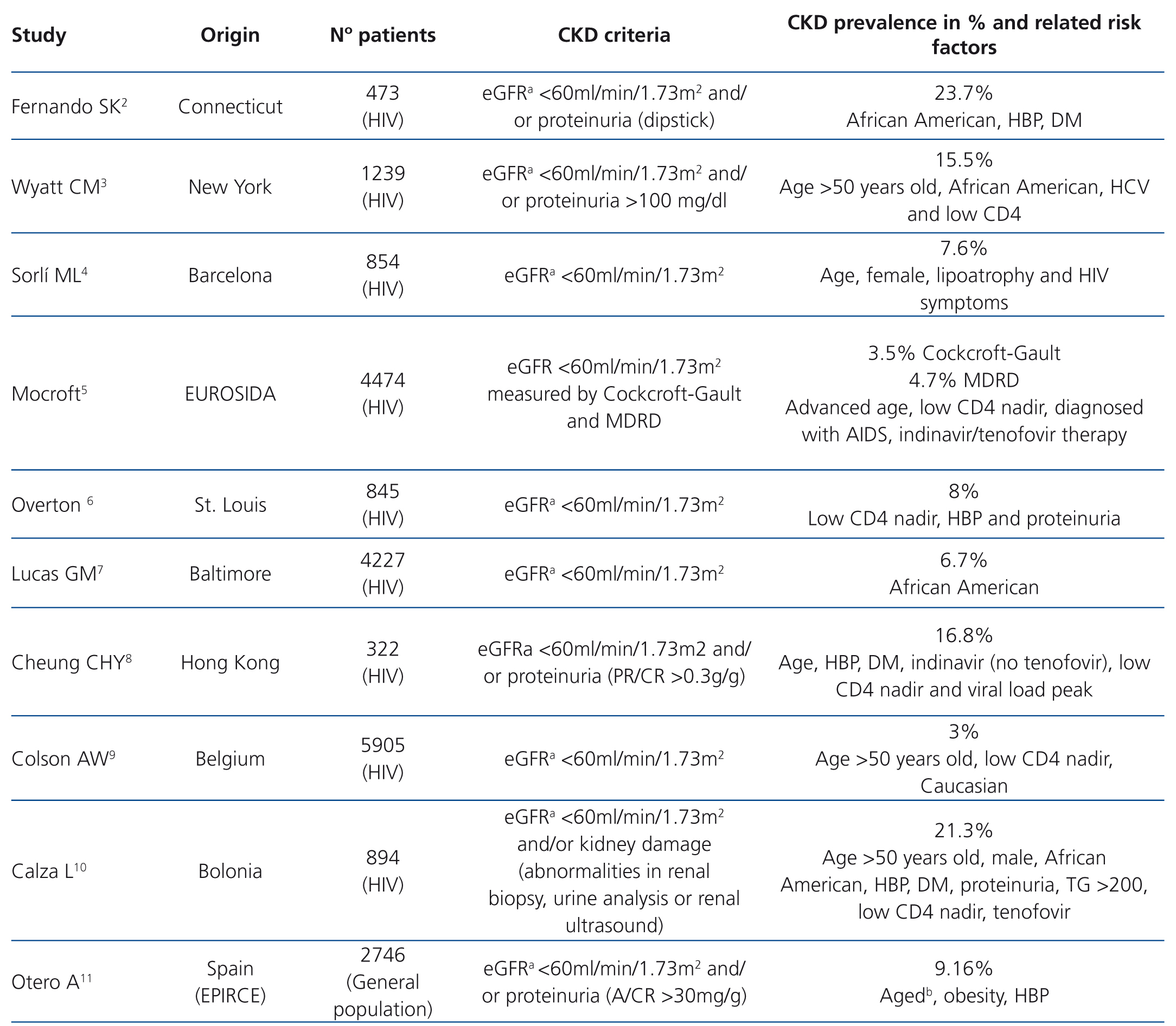
Antiretroviral therapy has dramatically improved the prognosis and survival of patients infected with the human immunodeficiency virus (HIV).1 This new situation has allowed pathologies to develop that in previous decades were considered to be less significant. Within this context, there is a growing interest in chronic kidney disease (CKD), and there are discrepancies both in terms of its prevalence and the factors involved in its development, including antiretroviral drugs (Table 1).2-11
With these objectives in mind (prevalence and risk factors), we reviewed patients treated at the Infectious Diseases Clinic of Zamora over a 6 month-period (October 2012-April 2013). Inclusion criteria: HIV infection, with at least two consecutive visits. Patients with concomitant acute disease at the time of the visit and/or those with a follow-up period of less than three months were excluded. We reviewed their medical histories and recorded their age, sex, weight, body mass index, follow-up time, concomitant chronic diseases (diabetes mellitus [DM], high blood pressure [HBP], chronic hepatitis B and/or C virus), smoking status, creatinine, phosphorus, proteinuria (measured by the albumin/creatinine ratio [A/CR]), urinary sediment, current CD4 count, CD4 nadir, the presence of AIDS, HIV RNA, antiretroviral therapy and tenofovir therapy (current and/or previous).
Estimated glomerular filtration rate (eGFR) was calculated using the MDRD (Modification of Diet in Renal Disease) and CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equations, with six categories being assigned in accordance with the recommendations of the National Kidney Foundation. CKD was defined as a decrease in kidney function, expressed by a glomerular filtration rate of <60ml/min/1.73m2 and/or persistent proteinuria (A/CR >30mg/g) for at least 3 months.
For statistical analysis, we used the SPSS 11.5.1 software. The association study was carried out using Χ2, exact tests, the Student’s t-test or ANOVA and multivariate logistic regression.
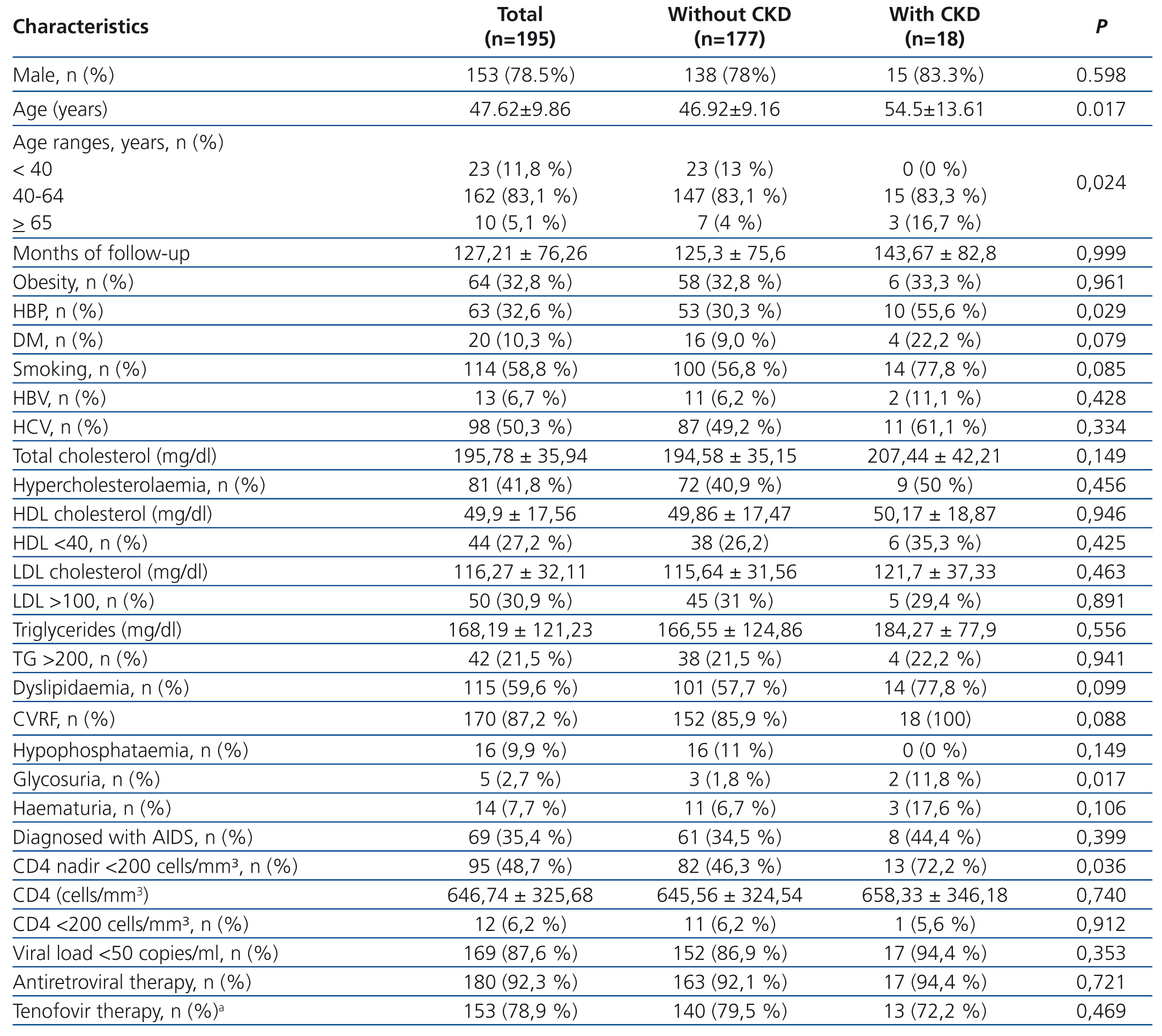
In accordance with the above mentioned criteria, we excluded 5 patients and included 195, whose epidemiological and clinical characteristics are displayed in Table 2. eGFR, calculated by MDRD, was 99.8±26.6ml/min/1.73m2, and by CKD-EPI, it was 98.4±18.4ml/min/1.73m2. The distribution by category was as follows: G1 124 patients (63.6%), G2 67 (34.4%), G3a 3 (1.5%), G3b 0, G4 0, G5 1 (0.5%) with MDRD, and G1 140 (71.8%), G2 52 (26.6%), G3a 2 (1.0%), G3b 0, G4 0, G5 1 (0.5%), with CKD-EPI. A total of 15 patients (7.7%) had proteinuria and 4 of them had an eGFR <60ml/min/1.73m2. On applying the MDRD formula, we found CKD in 18 (9.2%), and using CKD-EPI, in 17 (8.7%) (Table 2). Furthermore, 14 patients had microhaematuria, 5 had glycosuria (2 without DM) and 16 had hypophosphataemia. If we took into account any of these abnormalities, that is, eGFR <60 and/or proteinuria and/or microhaematuria and/or glycosuria and/or hypophosphataemia, 45 patients (23.1%) would be diagnosed with renal dysfunction. One or several cardiovascular risk factors (CVRF) were found in 87.2% and in 100% of those with CKD. Hyperlipidaemia and smoking were the most prevalent CVRF, followed by HBP and DM (Table 2). Differences in CKD prevalence were not found in patients with or without antiretroviral therapy, or between those treated and not treated with tenofovir (current and/or previous).
Variables associated with CKD were age, HBP and a low CD4 nadir (CD4 <200 cells/mm³) (Table 2). In the multivariate analysis, CKD was significantly associated with hbp (odds ratio [OR]: 3.1, p=.028) and a low CD4 nadir (OR=3.3, p=.03).
CKD prevalence was 9.2%, which is similar to that observed in the general Spanish population (9.16%).11 In patients infected with HIV, the data were conflicting, probably due to the lack of homogeneity in the criteria used for defining CKD. In Barcelona and in the EUROSIDA cohort, the results were similar to those expressed herein4,5 (Table 1).
In line with other publications, the data presented suggest that the development of CKD is associated with HBP and with a low CD4 nadir2,3,5,6,8-10 (Table 1). It was not observed that antiretroviral therapy or specifically tenofovir had a significant influence in this regard. We believe that this finding is particularly relevant, given its high use in this series.
We consider that these observations once again reveal similarities between those infected by HIV and the rest of the population: similar CKD, with HBP as the main risk factor. Its control, as with the rest of patients, seems to be essential in preventing CKD development. Furthermore, as has been demonstrated in many studies, poor immunity (low CD4 nadir) implies a worse prognosis and facilitates the development of many complications, among which we should probably include CKD.
The results presented suggest that CKD development in patients infected with HIV depends on two modifiable factors: low CD4 nadir and HBP. The control of both should be a main target in the daily work, and our task decisive.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Prevalence of chronic kidney disease in different cohorts and related risk factors.
Table 2. Demographic and clinical characteristics and comorbidities (the estimated glomerular filtration rate was calculated by MDRD).