Dear Editor,
The ability to become and remain pregnant in patients with chronic kidney disease depends on its stage. In early stages of the disease, there are practically no differences from a normal pregnancy.1 On the other hand, the difficulties that pregnancy poses to renal replacement therapy (RRT) are well known, and better outcomes have been described in patients who have undergone renal transplantation.2 However, the presence of advanced chronic kidney disease (stage 3-4) and pregnancy is an uncommon occurrence. Here we present the progression and treatment of a pregnant woman with stage 4 chronic kidney disease, which is especially unusual.
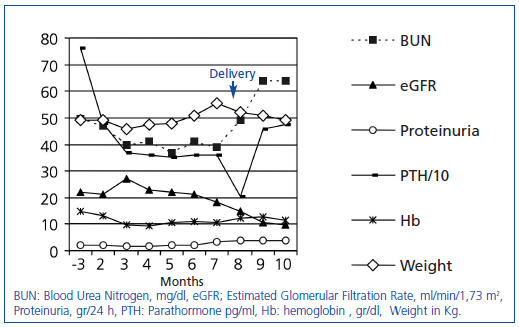
The patient is a 23-year-old female with epilepsy and chronic renal failure secondary to interstitial nephropathy. She was not hypertensive and presented, at one month of gestation, with the following laboratory findings: Hb: 13.1g/dl, Cr: 2.7mg/dl, urea: 101mg/dl, Ca: 9.1, P: 3.8mg/dl, HCO3: 19mmol/l, PTH: 480pg/ml, estimated glomerular filtration rate (eGFR) (MDRD- 4): 21ml/min/1.73m2, proteinuria: 2.23g/day; other tests without significant abnormalities. Weight 45.8kg and blood pressure (BP) 113/75mmHg. The progression of laboratory values can be seen in Figure 1. Clinical progression, BP control, presence of urea less than 100mg/dl or serum creatinine less than 4mg/dl, and ultrasound follow-up were established as the parametres to be assessed at the beginning of the RRT. These values remained within established limits throughout the pregnancy, with acceptable foetal progression until the eighth month. At that time, increased blood pressure (136/91), the presence of oedema, significant weight gain, and a mild increase in creatinine were noted. At 34+4 weeks, induction of labour was decided on for intrauterine growth restriction. During her admission she required treatment with labetalol due to increased BP. No haematologic or hepatic abnormalities were evident at any point. The neonate weighed 1,640g (3-10 percentile) and had respiratory distress consistent with hyaline membrane disease. Later, the neonate showed signs of persistent ductus arteriosus that required surgical closure. At 23 days of age, the infant was discharged with progressive weight gain and normal development to date. Three months after delivery, the mother was asymptomatic, with BP: 139/89, weight: 49.3 and Hb: 11.4, Cr: 5.5, urea: 137, and eGFR: 9, awaiting RRT initiation.
The association of advanced chronic kidney disease (CKD) and pregnancy is a rare event, with an incidence between 0.002 and 0.01% depending on the series.3 Decreased fertility, and the general tendency to discourage pregnancy in these stages, result in this low incidence.4 In turn, it is accepted that pregnancy at early stages, with eGFR greater than 60ml/min/1.73m2, does not alter the course of the renal disease and foetal viability is similar to women without chronic kidney disease.1 Outcomes in more advanced stages are not as clearly defined. In the largest collected series of 49 women with advanced CKD, stage 3-4, it was found that eGFR less than 40ml/min/1.73m2 and proteinuria greater than 1g/day at the start of pregnancy led to greater reduction in renal function and increased foetal morbidity and mortality.4 In another series, up to 10% of patients progressed to ESRD after pregnancy.5 On the other hand, estimation of renal function in pregnant women is not well defined. It is accepted that the formulas for estimating glomerular filtration rate are not adjusted for this cases6 and, at the same time, there are no clear indications for starting RRT in these situations. Some authors have set serum creatinine values between 3.5 and 4mg/dl for starting RRT.1 Other more recent studies in patients on haemodialysis have reported better results, with urea levels less than 100mg/dl.7,8 Although there is no firm evidence to support this, those values were set as limits in our patient. Renal function deteriorated, but did not exceed the limits that had been set, and thus it was not necessary to start RRT. At the same time, the presence of preeclampsia in these patients is also increased.4 However, the increase in BP and proteinuria in pregnant women makes it difficult to differentiate it from an exacerbation of the baseline disease.3 In our case we observed an increase in proteinuria and BP in the last trimester. The absence of hepatic or haematologic involvement could indicate a course related to the renal disease in the context of gestational changes.
From the neonatal perspective, although improvements in the paediatric intensive care have improved prognosis, the described mortality rate is between 4 and 4.9%, higher than in the normal population.4 The most common foetal complications are intrauterine growth restriction, low birth weight and preterm delivery.5 The association of proteinuria exceeding 1g/day and eGFR of less than 40 are risk factors for development.4 In our case there was intrauterine growth restriction in the final phase of pregnancy and low birth weight, complications associated with high morbidity. The presence of hyaline membrane syndrome and persistent ductus arteriosus is associated with premature birth, but we are unable to establish that role that renal disease plays in their development. In short, pregnancy is uncommon at stages 3-4; the association of proteinuria and an advanced renal stage implies a greater likelihood of renal disease and foetal morbidity. The approach that should be followed is based on recommendations and, although no guidelines exist regarding this, it seems reasonable to set the described parameters for monitoring purposes.
Figure 1. Laboratory parameters progression over the follow-up period. Follow-up at 3 months after the beginning of pregnancy and then monthly.