Content
Haemolytic-uraemic syndrome (HUS) is a disorder of the microvasculature, clinically defined by microangiopathic haemolytic anaemia (negative direct Coombs test) and thrombocytopaenia, that mainly affects the kidneys and is manifested by haematuria, oliguria and renal failure.1
CLINICAL CASE
A 14 year-old female with no relevant history. After returning from a trip to Istanbul, she began suffering episodes of diarrheal stools of a mucosanguineous nature (14-15 stools/day), accompanied by vomiting, abdominal pain, generalised weakness and fever. After these symptoms persisted for five days, she started suffering haematuria and oliguria and came to our hospital where deterioration of the glomerular filtration rate was detected in the laboratory (creatinine: 1.62mg/dl), thrombocytopenia (84 000mm3) and haemolytic anaemia accompanied by hyperbilirubinaemia at the expense of direct bilirubin (DB) (haemoglobin: 10.6g/dl, DB: 2.3mg/dl); coagulation tests were normal. When HUS was suspected, a new laboratory test was performed using a blood smear; severe deterioration was noted both clinically and in the laboratory: creatinine 2.6mg/dl, platelets: 39 000mm,3 haemoglobin 7g/dl (transfusion of 2 units of packed red blood cells was required). The presence of schistocytes in the blood smear was notable, and the stool test was negative for Shiga toxin.
An immunological study was performed with immunoglobulins, complements, anti-neutrophil cytoplasmic antibodies, anti-nuclear antibodies and anti-glomerular basement membrane antibodies, which was negative, thus ruling out systemic disease.
This data resulted in the diagnosis of HUS and support measures began with fluid therapy and plasmapheresis sessions through the temporary right femoral catheter, which was added to prednisone therapy (1mg/kg/weight).
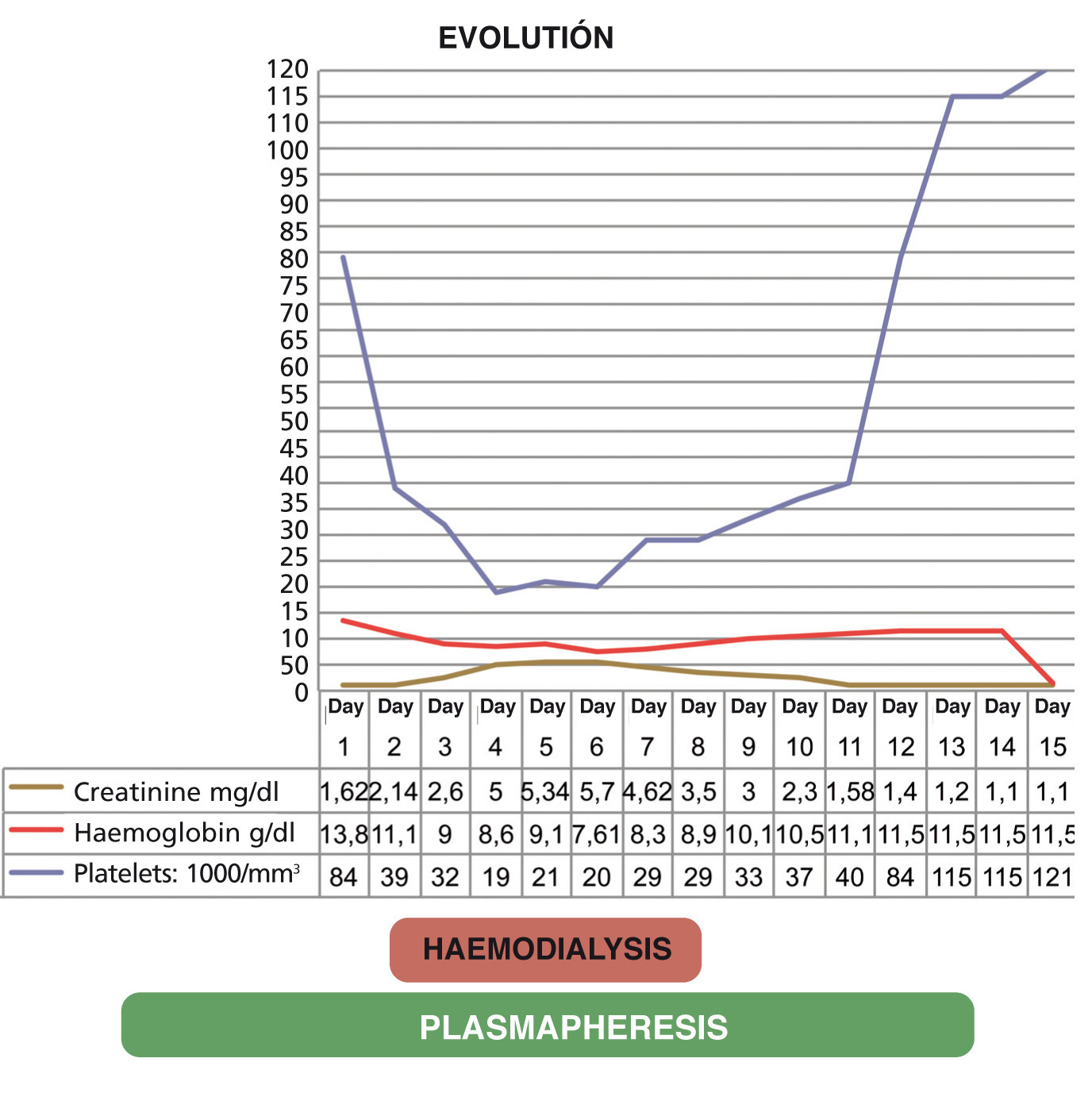
During the first seven days, evolution was slow, there was no response to daily plasmapheresis sessions and symptoms increased, beginning with an abrupt drop in the platelet count (19 000/mm3) and acute renal failure with anuria (renal function: creatinine 6 mg/dl). Three haemodialysis sessions were necessary.
In the eleventh session of daily plasmapheresis, there is a progressive increase in the number of platelets (86 000/mm3), an improvement in renal function (creatinine 1.5mg/dl) and increased diuresis (3000cc/24h), the haemodialysis sessions were therefore suspended.
Given the great clinical and laboratory test improvement, it was decided to continue with plasmapheresis sessions on alternate days with a progressive improvement both in renal function (until it normalised [creatinine 1.1mg/dl]) and haemolytic anaemia (haemoglobin: 9.6g/dl) and an increase in the platelet count (platelets: 115,000/mm3); diuresis was 2500cc/24h. The evolution of the patient is summarised in Figure 1.
The patient is currently clinically asymptomatic and normotensive with laboratory parameters (renal function, haemoglobin and platelets) being normal.
DISCUSSION
The HUS is a constellation of signs and symptoms characterised by the triad of microangiopathic haemolytic anaemia, thrombocytopaenia and acute renal failure.
This disorder may be divided into two forms in accordance with the association or non-association with bacteria that produce the Shiga-like toxin, as well as the clinical presentation:
- Typical HUS or HUS associated with the Shiga toxin (Stx) is the most common form of HUS (occurring in 90% of cases). It is caused by infection transmitted by food containing Shiga Toxigenic Escherichia coli. It usually occurs after an often bloody prodromal diarrhoea episode. It is usually self-limiting and normally has a benign course.2
- Atypical HUS or HUS not associated with Shiga toxin accounts for 10% of cases of HUS. The disease may occur sporadically (<20% of cases) or run in families. It is a heterogeneous group of disorders characterised clinically by the absence of diarrhoea in 92% of cases and a worse prognosis. Most patients have recurrences and over 50% develop stage 5 chronic kidney disease.3
The diagnosis to establish the association between HUS and infection by Shiga Toxigenic Escherichia coli (STEC) is based on three criteria: isolation and characterisation of the pathogen, detection of free faecal Stx and anti-Stx antibodies in serum.4
One of the limiting factors in applying all new and specific treatments is that the timeframe between diagnosis infection due to STEC and the applicability of the latter is very narrow. The diagnosis of the infection due to STEC should be carried out within 48 h of the time when diarrhoea began.5
In our case, it was not possible to confirm diagnosis, since the patient came in six days after the symptoms began; however, given that the symptoms suggested infection by E. coli (abdominal pain and bloody diarrhoeal stools) along with thrombocytopenia, microangiopathic haemolytic anaemia (high LDH and decreased haemoglobin) and the onset of acute renal failure, as well as good patient evolution, we confirmed that this was a case of typical HUS.
When HUS is suspected, it is essential to start treatment immediately, since it usually progresses and causes irreversible renal failure, progressive neurological deterioration, cardiac ischaemia and even death.
In conclusion, we insist that the best way to reduce cases of HUS is prevention: more stringent controls at all points of the food chain that ensure compliance with all food science laws and regulations at all levels, along with educational policies for the population as a whole.5
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Graph displaying evolution