The use of phosphate binders is one of basic elements in the treatment of CKD-MBD (“Chronic Kidney Disease-Mineral and Bone Disorder”).1
It has been well known for decades that phosphate retention through direct and indirect mechanisms, contributes to the generation and progression of secondary hyperparathyroidism (SHP) and renal osteodystrophy in chronic kidney disease (CKD).1,2 In fact, phosphate retention blocks all hormonal counter-regulatory mechanisms, increases skeletal resistance to PTH and is considered a central factor in the physiopathology of other endocrine and systemic alterations such as the decrease in calcitriol, increase in fibroblast growth factor-23 (FGF-23), cardiovascular calcifications, premature aging and the high morbi-mortality of these patients.2,3 For all this, phosphate has been considered as “the silent killer” of patients with CKD.4
As a consequence, especially in dialysis patients, the recent KDIGO guidelines underline the importance of dietary phosphate restriction, an adequate dose of dialysis and the use of phosphate binders.2 The evidence about the positive effect of the diet is limited and of low quality5 and an aggressive phosphate restriction is not only difficult but could also compromise the patient's nutritional status, counterbalancing the benefit of controlling phosphorus.6 In fact, the use of phosphate binders would allow a more liberal diet and a decreased risk of malnutrition.6
Although, by definition, all phosphate binders decrease the serum levels of phosphate, the characteristics of phosphate binders (with calcium, without calcium, with metals such as magnesium, iron or aluminum; polymers), in monotherapy or combination, seem to condition differential effects on a variety of aspects of the CKD-MBD complex (i.e. arterial calcification, FGF-23 levels) or on the survival of dialysis patients.7,8 The new KDIGO 2017 guidelines have increased the degree of evidence about the need to restrict the use of calcium containing phosphate binders in all CKD patients (evidence 2B)2 and this restriction does not only apply to those patients with hypercalcemia, arterial calcification, adynamic bone disease or patients with parathormone (PTH) persistently reduced, as suggested in previous guidelines. Likewise, it is still considered reasonable to assess the presence of vascular/valvular calcifications in patients with CKD to guide pharmacological management.2,9
Binders: adherence and pharmacological interactionsNephrologists are generally aware that patients with CKD, especially on renal replacement therapy, have to take a large number of drugs and a lot of them are phosphate binders in form of tablets/capsules/daily sachets.10 Simultaneous administration of several medications to the same patient is very frequent. Although this situation may be beneficial to improve therapeutic adherence, there are some drawbacks, as the possibility of pharmacological interactions, especially in patients of advanced age or with chronic pathologies.
An aspect often forgotten by nephrologists is that phosphate binders, due to its different biochemical properties, may have a marked differential influence on the absorption and effectiveness of many drugs. In fact, its simultaneous administration with other drugs is frequent and its possible clinical implications may be frequently unnoticed. Therefore, we believe it is important to improve this practice and review the pharmacological interactions described on the different phosphorus binders.
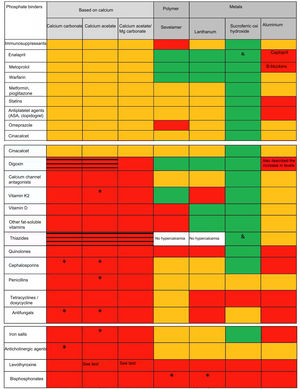
Pharmacological interactionsThe different effect of phosphate binders on the absorption of other drugs implies a pharmacokinetic interaction (generally affecting the absorption) which is different from pharmacodynamic interactions (affecting the binding to receptors or changing mechanisms of signal transduction). There are many reviews and tables that summarize the different effects of phosphate binders (dose, number of tablets, side effects, levels of calcium, phosphate, PTH, FGF-23 and even on the progression of vascular calcification and/or survival/mortality)9; however, there are not publications that analyzed and compared possible drug interactions such as the ones summarized in figure 1. It can be seen that most drug interactions are observed with calcium and aluminum containing phosphate binders.
Pharmacological interactions of major nephrological significance of the different phosphate binders available in Spain.
# See text. Aluminum hydroxide may increase the concentration of valproic acid (although its clinical significance is not established) and intoxications have been described for quinidine and digoxin. In addition to those shown, it reduces absorption or decreases levels of allopurinol and sucralfate, NSAIDs, carbenoxolone, chlorpromazine, epoetin, ketoconazole, ethambutol, gabapentin, isoniazid, metronidazole, penicillamine, ranitidine, chloroquine, cyclins, diflunisal, sodium fluoride, glucocorticoids, kayexalate, lincosamides, phenothiazines and neuroleptics, cefpodoxime, isoniazid and nitrofurantoin. It should be mentioned the decrease in corticosteroid absorption, although with doubtful clinical repercussions if the response to the drug is monitored. It is known that toxicity is increased by sodium citrate and vitamin C. It increases the excretion of acetylsalicylic acid and may alter the distribution of sodium pertechnetate in radioimaging tests.
Reduced absorption/reduced efficiency Increases the risk of hypercalcemia and secondary pharmacological toxicity (i.e. digitalis). In white, neither Sevelamer nor lanthanum have been studied with diuretics, but hypercalcemia is not a secondary effect. No interaction (demonstrated in in vitro or in vivo studies). Unspecified interaction. Not described but with a potential class effect. Demonstrated the absence of interaction also with furosemide and losartan.Calcium carbonate can modify the absorption of various drugs, so these should be administered at least 2h before or 4–6h after the binder. In addition to those shown, it should be highlighted other antibiotics such as neomycin, chloramphenicol and fosfomycin, systemic corticosteroids, phenytoin, barbiturates, antacids containing aluminum salts, cholestyramine, zinc, fluorides and iron salts.11,12 This last interaction is important to avoid combinations of the commonly used calcium-based phosphate binders8 with binders containing iron. As observed with the other binders it is important to remember that the effectiveness of levothyroxine can be reduced by the concurrent use of calcium binders. This means that the administration of calcium carbonate and levothyroxine should be separated by at least 4h.13
Another interaction to take into account, this one of a pharmacodynamic nature, is that hypercalcemia can increase the toxicity of digitalis (digoxin) and that thiazide diuretics reduce urinary calcium excretion, so there is an increased risk of hypercalcemia when coadministered with calcium-containing phosphate binders. Similarly, the concomitant treatment with vitamin D derivatives and/or medications or nutrients that contain calcium (milk) may favor hypercalcemia and the milk-alkaline syndrome. Besides, the intake of high amounts of calcium may cause a precipitation of bile and fatty acids in the form of soaps that could alter the absorption of ursodesoxycholic and chenodesoxycholic acid, as well as fats and fat-soluble vitamins.12,13
Calcium acetate (Royen®, RenaCare® calcium acetate)As mentioned for calcium carbonate, the administration of calcium acetate with some medications can alter its absorption or favor their toxicity through hypercalcemia. In addition to the information shown in the table, it should be highlighted the scarcely known effect on the impairment of calcium antagonists effectiveness.14 Despite the fact that a retrospective pharmacoepidemiological study had referred to the little or no interference of calcium acetate with the absorption of levothyroxine,15 recent data suggest that hypothyroid patients should be warned of taking the dose of levothyroxine clearly separated from any formulation with calcium.16
Calcium acetate/magnesium carbonate (Osvaren®)The combination calcium acetate/magnesium carbonate may alter the absorption of some medications included in the table, so they should not be taken within 2h before or 3h after the administration of the phosphate binder.17 In addition to the decrease in the absorption of cefuroxime, cefpodoxime or nitrofurantoin, it is also described the interaction with the absorption of zinc, fluorides and the antimalarial halofantrine.17 Likewise, the ingestion of magnesium can influence the absorption of iron, a common supplement included in the polymedication of the patient with CKD. Calcium acetate/magnesium carbonate may also produce hypermagnesemia, so antacids that contain not only calcium but also magnesium salts should be avoided. Magnesium salts may favor the absorption of digoxin in the gastrointestinal tract, decreasing its bioavailability, in addition to the possibility of increasing its potential toxicity due to hypercalcemia. The summary of product characteristics of this combination describes the possibility that concomitant use with estrogens may produce an increase sin serum calcium levels.17 Interestingly, unlike the other binders, it is mentioned that an increase in the absorption of levothyroxine may occur if given in combination with aluminum hydroxide17,18; however, as mentioned, recent data suggests that the administration of levothyroxine should be clearly separated from any formulation with calcium16 and other binders.
Binders without calcium: polymers and metalsAs shown in figure 1, the number of pharmacological interactions described with binders of a polymer structure (sevelamer) or metals (lanthanum, iron) is lower than that of calcium-based phosphate binders. However, there is abundant information regarding drug interactions of these binders as they were incorporated more recently to the therapeutic arsenal and these drugs had to address more queries to the official agencies in their clinical trials. We emphasize that in these studies, patients on antiarrhythmic or anticonvulsant medications were specifically excluded. In general, it would also be advisable to avoid co-administration of these drugs with any type of phosphate binders.
Sevelamer (Renagel®, Renvela®, generic Sevelámero)Sevelamer is a nonabsorbable cross-linked polymer. Medications that show a reduction of their bioavailability by sevelamer should be given at least one hour before or three hours after the administration of sevelamer.19 Studies on drug interactions in healthy volunteers revealed that sevelamer hydrochloride reduced the bioavailability of ciprofloxacin by approximately 50%.20 It is very important to know that sevelamer can reduce plasma levels of cyclosporine, tacrolimus and mycophenolate mofetil in transplant patients although without apparent clinical consequences (for example, graft rejection). Still, monitoring of their plasma levels is advisable during the use of this combination and after its withdrawal. TSH levels should be monitored in patients receiving levothyroxine (as with any binder). Sevelamer can decrease the absorption of fat-soluble vitamins D, E, K and folic acid, with debatable clinical repercussion.19 On the contrary, in healthy volunteers, sevelamer had no effect on the bioavailability of drugs as important as digoxin, warfarin, enalapril or metoprolol.21 However, coadministration of proton pump inhibitors with sevelamer can increase, rarely, serum phosphate levels.21
Lanthanum carbonate (Fosrenol®)Lanthanum may increase gastric pH (a similar effect is seen with calcium-based phosphate binders which are often used in combinations of antacids) and decrease the oral absorption of weak alkaline drugs. It is recommended not to take these compounds two hours before or after the administration of lanthanum carbonate (i.e. antimalarials such as chloroquine and hydroxychloroquine, the antifungal agent ketoconazole or bisphosphonates).22 The bioavailability of oral ciprofloxacin decreased by approximately 50% when administered together with lanthanum carbonate in a study on healthy volunteers.23 In contrast, lanthanum carbonate did not affect the serum concentrations of the liposoluble vitamins A, D, E and K,24 although recently in vitro interactions have been described with vitamin K2.25 It is also important to emphasize that, in healthy volunteers, the administration of lanthanum carbonate did not modify the pharmacokinetic profile of digoxin, warfarin, metoprolol, phenytoin or enalapril.22
Sucroferric oxyhydroxide [(OHS), Velphoro®]Unlike other phosphate binders, there is considerable information about the absence of OHS interactions with other drugs (see figure 1). Moreover, it has been described that there is no relevant interaction in vitro of OHS with cephalexin, nifedipine and quinidine, amongst others (figure 1).26 In healthy volunteers there are no relevant interactions either with losartan, furosemide, omeprazole, digoxin or warfarin.27 OHS does not interact with statins (atorvastatin and simvastatin) in dialysis patients, despite preliminary data in vitro suggested the possibility of such interaction. Unlike sevelamer, OHS does not affect the inhibitory activity of oral vitamin D upon PTH28 and, unlike lanthanum, does not seem to interact with Vitamin K2.25 It may be possible to see a lower oral absorption of drugs that interact with iron (such as alendronate and doxycycline) or, like the other binders, with levothyroxine.29
Aluminum-based bindersAlthough KDIGO guidelines recommend (evidence 1C) to avoid the prolonged use of aluminum-based phosphate binders,2figure 1 presents the multiple possibilities of interactions either by its potential prescription outside our scope or as a master formula.30 We must also highlight the possibility of unknown interactions with immunosuppressants or recently developed drugs.
ConclusionApart from the described potential negative effects of calcium-containing phosphate binders, we should also be taking into account the greater frequency of possible drug interactions with many drugs routinely used in our polymedicated patients. In general, other binders such as sevelamer, lanthanum and OHS have proven the absence of significant interactions with drugs for which there is no information available with the older binders. Likewise, it should be kept in mind that those binders who need a smaller number pills in monotherapy, will not only improve adherence to treatment but will also decrease the risk of possible undesirable interactions. In any case, although the potential for interactions seems low for some binders, especially OHS, its use with drugs with a narrow therapeutic range would advise to separate the doses to achieve the clinical effect and reduce adverse reactions, both at the beginning of treatment and after subsequent dose adjustments.
Declaration of the authorsThe authors approve the submission of the article for publication in the Nefrologia journal and declare that it has not been sent simultaneously to any other journal for publication. The authors also declare that it is an idea not originated in the pharmaceutical industry and that its intellectual property is transferred to Nefrologia. The authors approve the final form and are responsible for its content.
Conflicts of interestJ.B. has received lecture fees from Abbvie, Amgen, Genzyme and Shire, and as consultant from Abbvie, Amgen, Vifor/Fresenius-Renal Pharma, Chugai, Medice and Genzyme/Sanofi. J.F.N.G. has received lectures and/or consultancies fees from Abbvie, Amgen, Astra-Zeneca, Boehringer-Ingelheim, Esteve, Genzyme, Sanofi, Servier, Shire and Vifor/Fresenius-Renal Pharma. M.D.A. has received lectures or consultant fees from Shire, Fresenius, Abbvie and Amgen. E.G.P. has received consultant fees from Sanofi, Vifor/Fresenius-Renal Pharma, Amgen, Abbvie and Shire. A.L.M.F. has received lectures fees from General Electric and Astra Zeneca and consultant fees from Vifor/Fresenius-Renal Pharma. E.S. has received lectures fees from Abbvie, Sanofi, Shire, Vifor/Fresenius-Renal Pharma. A.M.M. has received lecture fees from Abbvie, Amgen, Shire, Bellco, Fresenius-Medical Care. M.J.Ll. has received lecture fees from Sanofi and Abbvie, and consultant fees from Vifor/Fresenius-Renal Pharma. P.M.V. has received lecture fees from Amgen, Sanofi and Vifor/Fresenius-Renal Pharma and consultant fees from Vifor/Fresenius-Renal Pharma. M.A.B. has received lecture fees from Vifor/Fresenius-Renal Pharma. I.D. has received fees for scientific collaboration with Vifor/Fresenius-Renal Pharma.