The clinical manifestations most commonly associated with the novel coronavirus disease (COVID-19) include fever, respiratory symptoms and digestive symptoms.1,2 However, as experience of the pandemic has grown, SARS-CoV-2 infection has been observed to cause cardiac, neurologic and renal damage, among others.3
Due to their immunosuppressed status, kidney transplant patients are considered a population at risk of developing more severe forms of the disease. Below, we describe a case of pericarditis secondary to COVID-19 infection in a kidney transplant recipient.
The patient is a 73-year-old male with chronic kidney disease of unspecified aetiology, who had a first kidney transplant in 2018, with baseline creatine around 1.2 mg/dl and immunosuppressant treatment with tacrolimus and mycophenolate mofetil (MMF).
He was admitted in March 2020 due to a clinical picture of cough, moderate effort dyspnoea and fever of 38°C of 4 days' evolution. On examination he presented bilateral crackles on auscultation, with no other findings of interest. In blood work, he presented reduced kidney function, with creatinine 1.57 mg/dl, C-reactive protein (CRP) 25.8 mg/dl, lymphopaenia 0.4 × 1000/mm3 and D-dimer 1051 ng/mL. His interleukin-6 (IL-6) value on admission was 32 pg/mL. An initial electrocardiogram did not show any alterations, while a chest X-ray showed diffuse bilateral opacities. RT-PCR for SARS-CoV-2 was performed on nasopharyngeal exudate with a positive result, so a diagnosis of pneumonia secondary to COVID-19 was established. Treatment was therefore started with hydroxychloroquine (200 mg/12 h for 12 days), lopinavir/ritonavir (200/50 mg/12 h for 5 days), and three 20-mg doses of 6-methylprednisolone. MMF was also suspended, while maintaining target tacrolimus levels at 5−6 ng/mL. The initial antibiotic prophylaxis included ceftriaxone, escalating to meropenem and linezolid at three days due to persistent dyspnoea and cough.
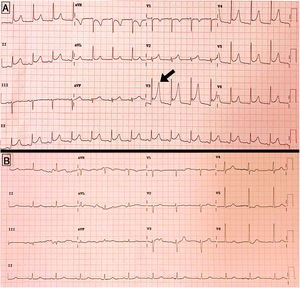
In spite of gradual respiratory improvement, on day +17 after admission, he began to experience atypical chest pain, and an electrocardiogram found a generalised PR segment depression with concave ST segment elevation in V3–V5, without elevated cardiac enzymes, all of which is compatible with acute pericarditis (Fig. 1A). At this time his D-dimer levels were 484 ng/mL, and PCR 3.7 mg/dl, but a new SARS-CoV-2 RT-PCR was not available. Table 1 shows the evolution of the main analytical parameters during the hospitalisation.
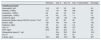
Evolution of the different analytical parameters during hospitalisation.
| Admission | Day +5 | Day +10 | Day +17 (pericarditis) | Discharge | |
|---|---|---|---|---|---|
| Analytical parameter | |||||
| Haemoglobin, g/dl | 10.6 | 10.7 | 9.2 | 9.8 | 9.5 |
| Platelets, ×1000/μl | 115 | 114 | 163 | 420 | 301 |
| Leukocytes, ×1000/μl | 8.6 | 4.8 | 5.4 | 5.2 | 8.5 |
| Lymphocytes, ×1000/μl | 0.4 | 0.8 | 0.5 | 1.1 | 1.2 |
| Creatinine, mg/dl | 1.57 | 1.29 | 1.22 | 1.42 | 0.99 |
| Glomerular filtration rate by CKD-EPI, ml/min/1.73 m2 | 43 | 55 | 58 | 49 | 75 |
| Sodium, mEq/l | 138 | 136 | 134 | 134 | 135 |
| Potassium, mEq/l | 3.8 | 4.4 | 4.8 | 4.5 | 4.5 |
| LDH, U/l | 242 | 292 | 350 | 270 | 357 |
| CRP, mg/dl | 25.8 | 9.8 | 22.1 | 3.7 | 3.6 |
| Ultrasensitive troponin T, ng/l | – | 24.4 | 26.3 | 26.6 | 14.2 |
| CK, U/l | – | 159 | 161 | 24 | 12 |
| D-dimer, ng/mL | 1051 | 998 | 1001 | 484 | 857 |
CK: creatine kinase; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CRP: C-reactive protein; LDH: lactate dehydrogenase.
The transthoracic echocardiogram found a preserved ejection fraction, with minimal posterior pericardial effusion and no haemodynamic compromise (Fig. 1B).
Treatment was started with colchicine 0.5 mg/12 h, with gradual disappearance of the chest pain and normalisation of the electrocardiographic changes.
Other complications during the hospitalisation included the need for oxygen for more than two weeks to maintain saturations above 94%, as well as the development of Clostridium difficile diarrhoea, which improved after 7 days’ treatment with vancomycin. For this reason, recovery of kidney function was slower than respiratory improvement and, consequently, the hospitalisation was prolonged. Finally, the patient was able to be discharged after a stay of one month.
This clinical case highlights that transplant recipients with SARS-CoV-2 infection can develop delayed cardiological complications of potential prognostic importance, such as pericarditis or myocarditis. It is therefore necessary to maintain a high degree of suspicion in order to correctly diagnose and treat these. To date, three cases of myo-pericarditis secondary to SARS-CoV-2 infection have been reported,4 with this case being the first in a kidney transplant recipient.
The prolonged duration of the hospitalisation in this case compared to the median length of stay reported in other series1,2 allowed earlier diagnosis of this complication.
It is known that certain viral infections can trigger pericarditis. It is thought that this complication might be mediated by direct cytotoxic damage by the virus, or develop through inflammatory mechanisms secondary to a “cytokine storm” in which IL-6 or TNF-α would play a fundamental role.5
It is known that SARS-CoV-2 uses angiotensin-converting enzyme receptors to infect cells.6 Although their expression is more abundant in lung tissue, they are also expressed on the cells of other organs such as the myocardium.4 In this sense, there is some debate around the possible deleterious effect of treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin-2 receptor blockers (ARB) in these patients; however, our case did not receive either of these drugs.
The therapeutic alternatives currently available for COVID-19 infection are few, while we await the availability of specific antiviral drugs.7 On the other hand, non-steroidal anti-inflammatories are the basis of treatment in pericarditis of infectious aetiology, with colchicine or even pericardiocentesis used in more severe cases.8
Complications of this type can prove a therapeutic challenge in kidney transplant recipients, given the pharmacological contraindications in this group of patients. In our case, it was decided to start treatment with colchicine, considering also its possible beneficial effect against SARS-CoV-2 described in experimental studies.9 Tolerance was good, without adverse effects, and the clinical course was favourable.
In conclusion, COVID-19 infection can trigger the onset of cardiac complications such as acute pericarditis in kidney transplant recipients. Treatment with colchicine would be effective in this group of patients, although further studies are needed that analyse the main risk factors for this complication, as well as the best treatment strategies.
Conflicts of interestThe authors declare they have no conflicts of interest related to the publication of this article.
Please cite this article as: Sandino Pérez J, Aubert Girbal L, Caravaca-Fontán F, Polanco N, Sevillano Prieto A, Andrés A. Pericarditis secundaria a infección por COVID-19 en un paciente trasplantado renal. Nefrologia. 2021;41:349–351.