The recognition of certain autoantibodies in the pathogenesis of membranous glomerulonephritis has enabled the use of anti-CD20 therapies. Some 70–80% of patients have serum autoantibodies to phospholipase A2 receptor (PLA2R). Treatment with rituximab has been shown to be at least as effective as cyclophosphamide; however, its use is associated with recurrences (27%), requiring repeated doses, and there is a lack of response in 35–45% of cases, necessitating treatments that are associated with short- and long-term toxicity.1,2
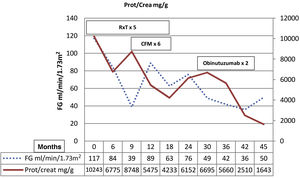
We present the case of a 44-year-old male who in November 2017 had severe nephrotic syndrome (total protein 4.9g/dl [6.0–8.0], albumin 2.5g/dl [3.5–5.0], urine protein/creatinine ratio 10.243.42mg/g), with normal renal function (urea 31mg/dl, creatinine 0.66mg/dl, estimated glomerular filtration rate CKD-EPI [eGFR] 117.6ml/min/1.73m2), and was diagnosed with membranous glomerulonephritis by renal biopsy, with presence of serum anti-PLA2R antibodies (positive 109.44 UR/ml). The secondary study of potential causes was negative.
Treatment was started with rituximab (induction 1000mg/15 days, 2 doses) and oral tacrolimus (with control of serum level), requiring rituximab bolus at the sixth month. At the ninth month of evolution, due to progressive deterioration of renal function and persistence of nephrotic syndrome, we withdrew tacrolimus and initiated treatment with cyclophosphamide in monthly boluses (6 boluses). We continued treatment with semiannual rituximab until 18 months of treatment (a total of 5 boluses), with persistence of renal function deterioration (urea 42mg/dl, creatinine 1.86mg/dl, GFR 42ml/min/1.73 m2), nephrotic syndrome (albumin 2.0g/dl, total protein 3.5g/dl, urine protein/creatinine 5660.65mg/g) and anti-PLA2R positivity (positive: 109.44 UR/ml).
In February 2022, we decided to treat the patient with obinutuzumab (100mg, 900mg on consecutive days, and 1g after 14 days), and observed a partial response in the sixth month (albumin 2.8g/dl, total protein 4.7g/dl, protein/creatinine 2510.46mg/g, urea 71mg/dl, creatinine 1.99mg/dl, GFR 38ml/min/1.73 m2), that was confirmed at the ninth month (albumin 3.1g/dl, total proteins 5.0g/dl, protein/creatinine 1643.19mg/g), with improvement of renal function (urea 46mg/dl, creatinine 1.59mg/dl, GFR 50ml/min/1.73 m2) and negativity of PLA2R antibodies. The patient had no infusion-related adverse effects or infectious complications after treatment (Fig. 1).
Rituximab is a chimeric anti-CD20 type 1 monoclonal antibody that upon binding to the CD20 receptor internalizes and degrades it, resulting in reduced macrophage recruitment and phagocytosis, with reduced B-cell elimination and increased antibody requirement.
Obinutuzumab is a humanized anti-CD20 type 2 monoclonal antibody with greater cytotoxicity than rituximab against B cells in vitro. It binds to a CD20 epitope different from rituximab, preventing internalization of the CD20/antibody complex. Due to a modification in its carbohydrate, it has a higher affinity for the Fcγ RIIIa of B cells, which translates into greater ability to recruit effector cells, greater direct cytotoxicity, with less reliance on complement-dependent cytotoxicity compared to rituximab. In addition, it induces direct lysosome-mediated cell death and less dependence on high levels of B-cell activating factor, which contributes to greater depletion of memory B cells, which are more resistant to the action of rituximab.
The clinical experience of obinutuzumab in membranous glomerulonephritis is limited to:
- -
Three patients with anti-PLA2R-positive membranous glomerulonephritis treated with rituximab, without clinical and immunological remission, and in whom, after treatment with obinutuzumab, complete immunological remission was achieved in all three patients, followed by improvement in proteinuria and normalization of serum albumin.3
- -
A sample of 10 patients with rituximab-resistant membranous glomerulonephritis, in whom, after treatment with obinutuzumab, complete remission was achieved in 40% and partial remission in 50%, with immunological remission in all cases and response maintained at 24 months.4
- -
Two patients with anti-PLA2R-positive membranous glomerulonephritis resistant to prednisone, cyclosporine, cyclophosphamide and rituximab, with immunological remission 12 months after treatment with obinutuzumab, with normalization of serum albumin, improvement of proteinuria and stable renal function.5
- -
A patient with membranous glomerulonephritis secondary to IgG4-related disease, with anaphylactic reaction to rituximab, with complete remission and normalization of IgG4 levels after treatment with obinutuzumab.6
In conclusion, obinutuzumab is a therapeutic alternative in patients with membranous glomerulonephritis in whom immunologic and clinical remission is not achieved with rituximab and avoids the need for treatments with greater toxicity. Currently there is a phase iii clinical trial to evaluate the efficacy and safety of obinutuzumab compared to tacrolimus in patients with primary membranous glomerulonephritis (ClinicalTrials.gov Identifier: NCT04629248).
Consent to publicationThe authors declare that the patient presented in this article has given consent to use his or her medical information for this publication.
FundingThis research has not received specific support from public sector agencies, commercial sector or non-profit entities.
Conflict of interestThe authors declare that they have no conflicts of interest.