Obinutuzumab is a type II humanized anti-CD20 monoclonal antibody of the IgG 1 isotype, initially used in B-cell neoplasms resistant to rituximab (RTX). Randomized studies are currently underway with good results compared to other anti-CD20 monoclonal antibodies in membranous glomerulonephritis (MGN) and lupus nephropathy (LN).1,2
We present the case of a 63-year-old male with a history of arterial hypertension, under follow-up at the nephrology outpatient clinic since 2015 for clinical nephrotic syndrome and the following lab results: proteinuria of 10 g/24 h, albumin 2.4 g/dl, cholesterol 308 mg/dl, urine systematics with protein +++ and dysmorphic red blood cells in addition to the presence of edema in the lower limbs.
We extended the glomerular study (autoimmunity, serology, complement, immunoglobulins) obtaining h negative results and normal renal function. We performed a renal biopsy in July 2015 with results compatible with minimal change glomerulonephritis (MCD).
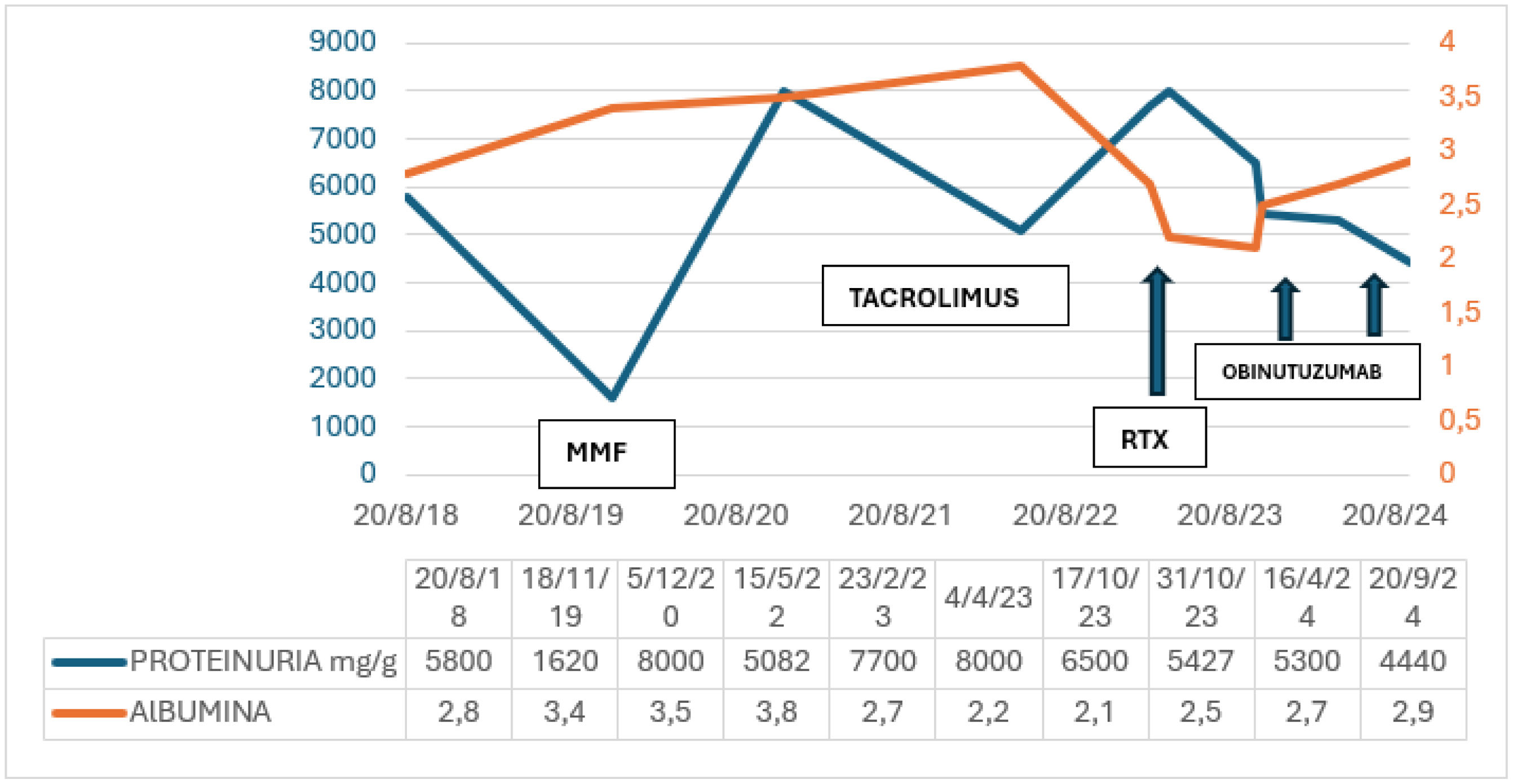
We first treated the patient with corticosteroid using prednisone at a dose of 1 mg/kg/day/for 16 weeks presenting with a partial response with proteinuria of 2.2 g/24 h. In December 2016, when trying to lower the prednisone dose to 5 mg/day, we detected a new flare of clinical nephrotic syndrome with worsening edema and proteinuria of 8.7 g/24 h and albumin 2.7 mg/dl and categorized the patient as corticodependent.
Then we restarted prednisone at a dose of 1 mg/kg/day and he presented partial improvement: decrease in proteinuria to 2.4–3.5 g/24 h in June 2017. In view of the patient's relapses and corticodependence and following KDIGO therapeutic guidelines, we decided to associate tacrolimus to the treatment from June 2017 to July 2018; the patient had minimum proteinuria of 2 g/24 h in the February 2018 control.2,3
Subsequently, the patient presented a new biochemical flare of the disease with proteinuria of 5.8 g/24 h and hypoalbuminemia, so we discontinued tacrolimus. We treated the patient again with corticosteroids and added cyclophosphamide at 100 mg/24 h/orally for 8 weeks.
In November 2019 the patient presented a new flare of nephrotic syndrome, which we treated with mycophenolate mofetil (MMF), as a fourth line of treatment, and proteinuria decreased to 0.5 g by May 2022. We suspended MMF in May 2022 due to macroscopic hematuria and left renal tumor. We performed a partial nephrectomy and found that the histological specimen was compatible with organized hematoma. The histological specimen justified referral again to anatomic pathology and to electron microscopy, given the poor evolution of the patient with MCD. Optical microscopy showed minimal change disease with extensive acute tubulointerstitial nephritis. Electron microscopy revealed focal segmental glomerulonephritis. We performed a genetic study and observed no associated mutations. With this result and after a new biochemical flare in October 2022 (proteinuria 8 g/day, active urine and albumin 2.2 mg/dl), we restarted MMF, later changing to tacrolimus, due to the lack of response. As proteinuria of 8 g/day persisted, in February 2023, we added to the immunosuppression RTX1 g in 2 administrations. There was no response: proteinuria 8 g/day and hypoalbuminemia persisted.
We reviewed the literature, and in October 2023 administered off-label the first dose of obinutuzumab of 1000 mg (in 2 administrations: 100 and 900 mg) and the second dose of 1000 mg 15 days later. With this treatment and in subsequent controls we observed a decrease in proteinuria to 4.4 g/day and inactivation of the urine system, and improved hypoalbuminemia of 2.9 mg/dl. Currently the patient presents partial improvement after the third dose of the drug and maintenance treatment with tacrolimus in descending doses (Fig. 1).
Our patient presents a nephrotic syndrome clinically characterized by edema in lower limbs, considerable loss of protein in urine and normal renal function. In other series investigators report corticosteroid resistance in 8%–25% of patients, with a lower prevalence in minimal change disease.3,4 Obinutuzumab has been used as salvage therapy in patients with MGN who do not achieve complete remission and in lupus nephropathy, however, its use in MCD and focal segmental glomerulonephritis is novel and there are few documented cases.5,6 This case stands out and focuses on the management of nephrotic syndrome and is unusual for its multiple relapses and partial responses to different immunosuppressants including RTX, a monoclonal antibody with extensive previous experience.7,8
Other series have attributed non-responses to RTX treatment to the absence of B lymphocyte repletion. In our case, the patient presented B lymphocyte depletion, but we did not observe a response after 6 months of treatment with RTX.8,9
Therefore, we switched to another anti-CD20 monoclonal antibody with a different, innovative mechanism of action, acting on a different CD20 target, recently used in resistant glomerulonephritis, with few cases described in the literature.
We highlight the use of obinutuzumab in patients with glomerular disease (minimal change disease and focal segmental glomerulonephritis) resistant to other anti-CD20 monoclonal antibodies, but only further studies can corroborate these results.