Patients with chronic kidney disease (CKD) develop bleeding and thrombotic tendencies, so the indication of anticoagulation at the onset of atrial fibrillation (AF) is complex. AF is the most common chronic cardiac arrhythmia, and thromboembolism and ischaemic stroke in particular are major complications. In recent years, new oral anticoagulant drugs have been developed, and they have shown superiority over the classical AVK in preventing stroke, systemic embolism and bleeding risk, constituting an effective alternative to those resources.
Los pacientes con enfermedad renal crónica (ERC) tienen tendencias hemorrágicas y trombóticas, por lo que la indicación de anticoagulación ante la aparición de fibrilación auricular (FA) es compleja. La FA es la arritmia cardíaca crónica más frecuente, siendo el tromboembolismo y el ictus isquémico en particular las complicaciones más importantes. En los últimos años se han desarrollado nuevos fármacos anticoagulantes orales que han mostrado superioridad respecto a los clásicos antagonistas de la vitamina K (AVK) en la prevención de ictus, embolismo sistémico y riesgo de sangrado, constituyendo una alternativa eficaz a ellos.
Patients with chronic kidney disease (CKD) presents a difficult problem in daily clinical practice; these patients have a bleeding tendency due to a primary disorder of haemostasis secondary to platelet dysfunction and an abnormality in platelet/subendothelial interaction1; the same patients also had a clotting tendency caused by multiple factors, such as endothelial damage, increased coagulation factors and decreased fibrinolytic proteins.2 Therefore, tin these patients the indication of anticoagulation therapy for atrial fibrillation (AF) is a complex decision, even more so because of the new oral anticoagulants that are now available and which are different in their renal pharmacokinetics.
The purpose of this article is to clarify decisions concerning whether to give anticoagulation to CKD patients who develop AF, and which anticoagulant drug is best in each case.
Epidemiology and predisposing factors of atrial fibrillation in chronic kidney diseaseAF is the most common chronic cardiac arrhythmia, and its prevalence in CKD is 10–20 times higher than in the general population.3 In fact, AF and CKD often coincide: one-third of AF patients have CKD, and 15% of CKD patients appear to have AF.4 In patients on haemodialysis studied with a Holter ECG monitor, the frequency of AF reaches up to 27%. No accurate data are available in CKD patients who are not on dialysis (Table 1).
Prevalence of atrial fibrillation.3
| General population <60 years: | 1% |
| General population <80 years: | 8% |
| Patients on peritoneal dialysis | 7% |
| Patients on haemodialysis | 13%–27% |
CKD patients are at a high risk of developing cardiovascular disease, and at the same time and in the opposite direction, the prevalence of CKD is greater in people with cardiovascular disease than in the general population, which is associated with a worse prognosis. In particular, vascular and valvular calcification, left ventricular hypertrophy, hydroelectrolytic disorders during dialysis and renin–angiotensin–aldosterone system (RAAS) hyperactivity are predisposing factors for AF. Recently, one study showed that RAAS inhibition with ACE inhibitors and/or angiotensin II receptor blockers (ARBs) is effective in the primary prevention of AF in patients on dialysis.5
Risk of stroke in chronic kidney diseaseThe most significant complications of AF are thromboembolism, particularly ischaemic stroke, which are usually more severe in terms of residual disability and short- and medium-term mortality.6 The incidence of stroke is higher among patients on haemodialysis than in the general population. In a study conducted in our nephrology department at Hospital Universitario Valdecilla, in Santander, the cumulative incidence of cerebrovascular events was 5.8%. The incidence rate during the first year on haemodialysis was 6.5% higher than the mean observed during the study period.7 Murray et al.8 observed that the number of strokes during the first month on haemodialysis increased by as much as 7 times. The same was observed in a European database of haemodialysis patients.9 The reasons for this higher incidence in the early stages of haemodialysis are not clear; suggested as possible factors are decreased cerebral perfusion and blood flow velocity, or, in patients treated with erythropoiesis-stimulating agents (ESA), increased viscosity and vascular resistance that can lead to increases in blood pressure. In the United States Renal Data System (USRDS), the incidence of stroke was 15.1% in haemodialysis, 9.6% in patients with CKD but not on haemodialysis and 2.6% in patients without CKD.10 In Jaén, Spain, a study by Vázquez et al.11 found a rate of thromboembolic complications of 24% per year in patients on dialysis in AF, compared with 5% in patients with sinus rhythm. In the USRDS,10 an 80% greater chance of ischaemic stroke is reported in AF patients with a similar incidence of haemorrhagic stroke. A stroke clearly increases mortality in CKD patients compared with those without CKD.3
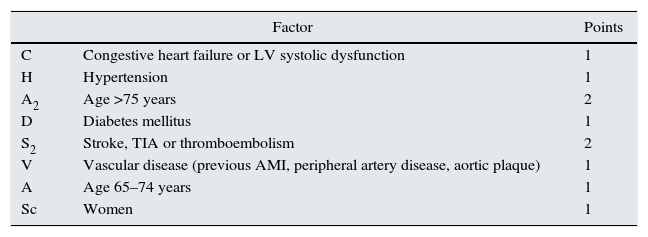
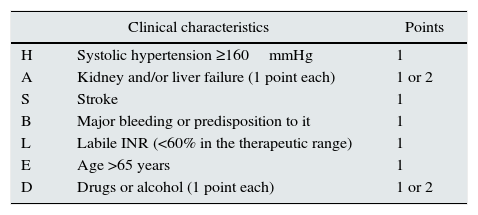
Risk of stroke in chronic kidney disease with atrial fibrillation, CHA2DS2-VASc score vs. risk of bleeding, HAS-BLED scoreAmong the factors associated with stroke, in our patients we found diabetes, myocardial infarction or angina pectoris, hypertension, arteriosclerosis/intermittent claudication and history of cerebrovascular accident before haemodialysis.7 However, we did not find AF to be an associated factor, as in other more recent works,12 but this may be due to the no differentiation of ischaemic stroke from haemorrhagic stroke. The American Heart Association's13 2014 clinical practice guidelines recommend (1B) using the CHA2DS2-VASc score to assess risk of stroke in patients with AF. The majority of kidney patients have a high risk of stroke14 (Table 2). Also, kidney patients have a high risk of bleeding according to the HAS-BLED score15 (Table 3).
CHA2DS2-VASc score for assessing the risk of stroke in atrial fibrillation.
| Factor | Points | |
|---|---|---|
| C | Congestive heart failure or LV systolic dysfunction | 1 |
| H | Hypertension | 1 |
| A2 | Age >75 years | 2 |
| D | Diabetes mellitus | 1 |
| S2 | Stroke, TIA or thromboembolism | 2 |
| V | Vascular disease (previous AMI, peripheral artery disease, aortic plaque) | 1 |
| A | Age 65–74 years | 1 |
| Sc | Women | 1 |
TIA: transient ischaemic attack; AMI: acute myocardial infarction; LV: left ventricle.
Maximum: 9 points. Low risk: 0; intermediate risk: 1; high risk ≥2.
Source: Camm et al.14
Estimation of bleeding risk: HAS-BLED.
| Clinical characteristics | Points | |
|---|---|---|
| H | Systolic hypertension ≥160mmHg | 1 |
| A | Kidney and/or liver failure (1 point each) | 1 or 2 |
| S | Stroke | 1 |
| B | Major bleeding or predisposition to it | 1 |
| L | Labile INR (<60% in the therapeutic range) | 1 |
| E | Age >65 years | 1 |
| D | Drugs or alcohol (1 point each) | 1 or 2 |
INR: international normalised ratio.
Maximum: 9 points. Score ≥3 indicates “high risk”.
Source: Pisters et al.15
In recent years, research has focused on the development of new oral anticoagulants (NOACs), with a wide therapeutic range, low variability, and which can be given at fixed doses, with no need for monitoring due to their short half-life and pharmacokinetic and pharmacodynamic properties, and with few interactions and a variable degree of renal excretion.
One of the main drawbacks of NOACs was the absence of specific agents capable to effectively reverse their anticoagulant effect. Idarucizumab,16 a new specific agent for reversal of dabigatran, has recently appeared. It is indicated for adult patients requiring rapid reversal of anticoagulant effects for emergency surgery or urgent procedures and in cases of potentially fatal or uncontrolled bleeding. For the rest of the NOACs, new specific reversal agents are expected soon.
The other major drawback is that NOACs are more expensive than vitamin K antagonists (VKAs), although their cost tends to decrease as new drugs arrive on the market, and with lower risk of thromboembolic events and risk of bleeding. They therefore provide a cost-effective alternative as compared with VKAs.
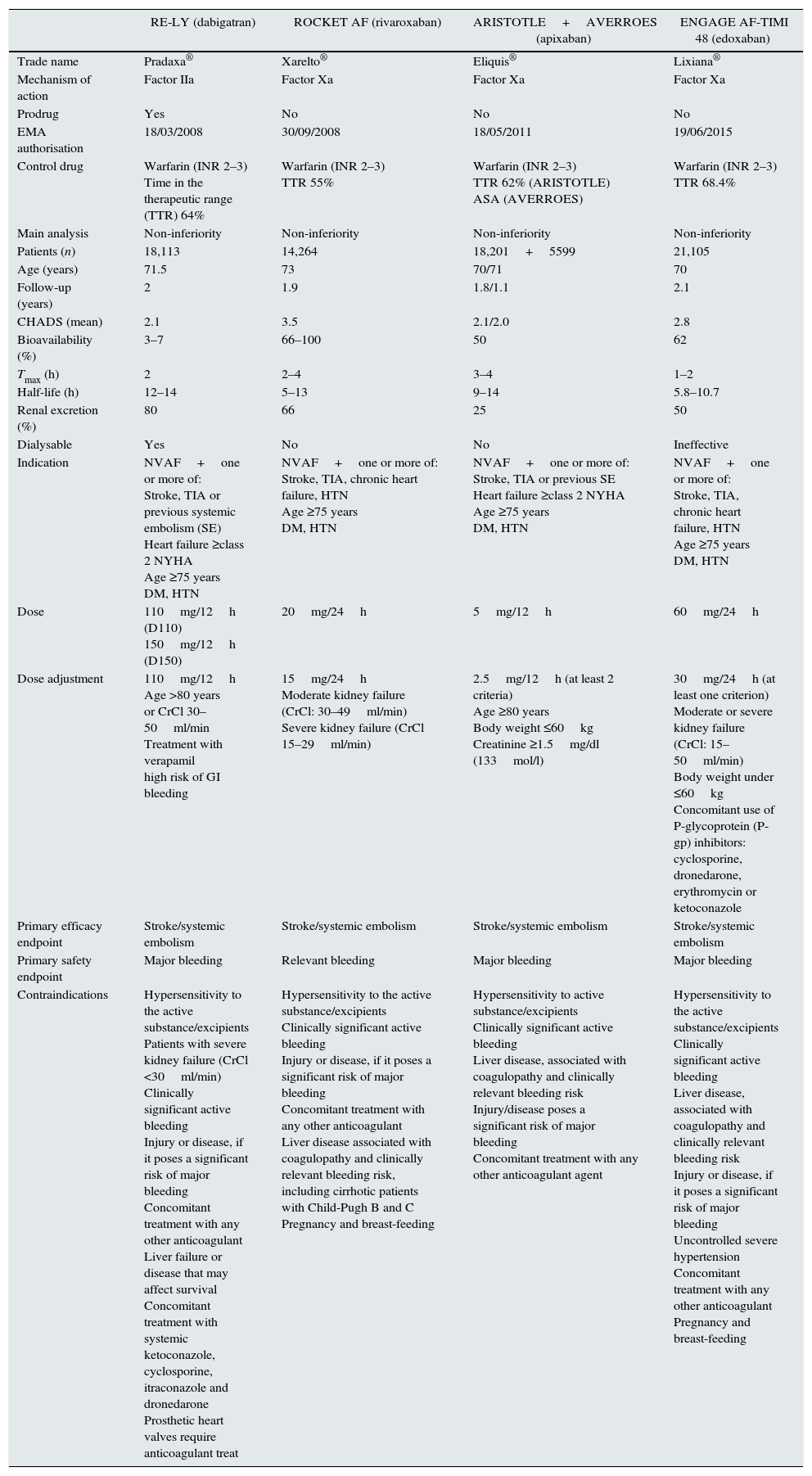
Presently there is a direct thrombin inhibitor (dabigatran) and 3 direct inhibitors of activated factor X (rivaroxaban, apixaban and edoxaban), with approved indications for prophylaxis and antithrombotic treatment in different situations with a favourable benefit/risk balance in various clinical conditions where anticoagulation is indicated.17 The use of these direct-acting NOACs is associated with benefits and drawbacks regarding the use of VKAs, but perhaps one of the most interesting aspects to be clarified is their use in patients with CKD.
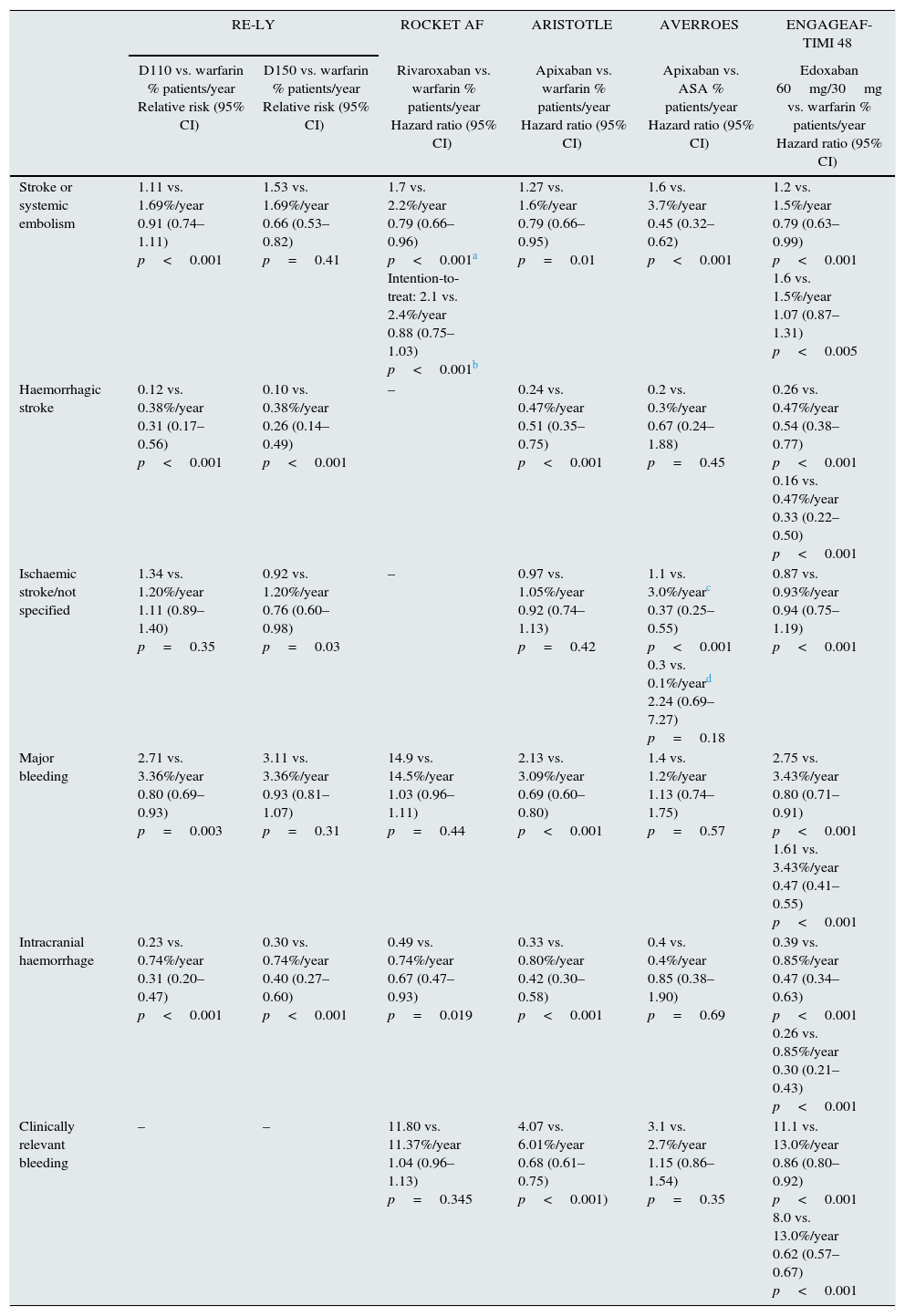
According to the RE-LY study,18 dabigatran at a dose of 110mg twice daily (D110) was shown to be superior to warfarin in preventing stroke and systemic embolism, with lower risk of bleeding. At a dose of 150mg twice daily (D150), it was superior to warfarin in reducing stroke or systemic embolism, with no significant differences in major bleeding and with an increased risk of gastrointestinal bleeding. In addition, here was a non-significant increase in myocardial infarctions with both doses of dabigatran. The ROCKET AF study19 indicates that, as compared with warfarin, rivaroxaban at a dose of 20mg was non-inferior in preventing stroke or systemic embolism; it also showed no differences in the risk of major bleeding, but there was less incidence of intracranial and fatal bleeding. The ARISTOTLE study20 shows that apixaban at doses of 5mg twice daily showed superiority as compared with warfarin in preventing stroke, systemic embolism and major bleeding. The AVERROES study21 showed the same superiority of apixaban as compared with acetylsalicylic acid (ASA).
Finally. according to the ENGAGE AF-TIMI 48 study,22 edoxaban at a dose of 60mg was non-inferior than warfarin in preventing stroke and systemic embolism. As far as the occurrence of major bleeding, as well as in intracranial and other types of bleeding, edoxaban (30/60mg) that significantly reduces the risk of bleeding as compared with the warfarin group. Tables 4 and 5 summarise the main features of the 4 primary studies showing clinical evidence of the efficacy of these NOACs.
Main characteristics of phase III clinical trials on embolism prevention in patients with non-valvular atrial fibrillation.
| RE-LY (dabigatran) | ROCKET AF (rivaroxaban) | ARISTOTLE+AVERROES (apixaban) | ENGAGE AF-TIMI 48 (edoxaban) | |
|---|---|---|---|---|
| Trade name | Pradaxa® | Xarelto® | Eliquis® | Lixiana® |
| Mechanism of action | Factor IIa | Factor Xa | Factor Xa | Factor Xa |
| Prodrug | Yes | No | No | No |
| EMA authorisation | 18/03/2008 | 30/09/2008 | 18/05/2011 | 19/06/2015 |
| Control drug | Warfarin (INR 2–3) Time in the therapeutic range (TTR) 64% | Warfarin (INR 2–3) TTR 55% | Warfarin (INR 2–3) TTR 62% (ARISTOTLE) ASA (AVERROES) | Warfarin (INR 2–3) TTR 68.4% |
| Main analysis | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority |
| Patients (n) | 18,113 | 14,264 | 18,201+5599 | 21,105 |
| Age (years) | 71.5 | 73 | 70/71 | 70 |
| Follow-up (years) | 2 | 1.9 | 1.8/1.1 | 2.1 |
| CHADS (mean) | 2.1 | 3.5 | 2.1/2.0 | 2.8 |
| Bioavailability (%) | 3–7 | 66–100 | 50 | 62 |
| Tmax (h) | 2 | 2–4 | 3–4 | 1–2 |
| Half-life (h) | 12–14 | 5–13 | 9–14 | 5.8–10.7 |
| Renal excretion (%) | 80 | 66 | 25 | 50 |
| Dialysable | Yes | No | No | Ineffective |
| Indication | NVAF+one or more of: Stroke, TIA or previous systemic embolism (SE) Heart failure ≥class 2 NYHA Age ≥75 years DM, HTN | NVAF+one or more of: Stroke, TIA, chronic heart failure, HTN Age ≥75 years DM, HTN | NVAF+one or more of: Stroke, TIA or previous SE Heart failure ≥class 2 NYHA Age ≥75 years DM, HTN | NVAF+one or more of: Stroke, TIA, chronic heart failure, HTN Age ≥75 years DM, HTN |
| Dose | 110mg/12h (D110) 150mg/12h (D150) | 20mg/24h | 5mg/12h | 60mg/24h |
| Dose adjustment | 110mg/12h Age >80 years or CrCl 30–50ml/min Treatment with verapamil high risk of GI bleeding | 15mg/24h Moderate kidney failure (CrCl: 30–49ml/min) Severe kidney failure (CrCl 15–29ml/min) | 2.5mg/12h (at least 2 criteria) Age ≥80 years Body weight ≤60kg Creatinine ≥1.5mg/dl (133mol/l) | 30mg/24h (at least one criterion) Moderate or severe kidney failure (CrCl: 15–50ml/min) Body weight under ≤60kg Concomitant use of P-glycoprotein (P-gp) inhibitors: cyclosporine, dronedarone, erythromycin or ketoconazole |
| Primary efficacy endpoint | Stroke/systemic embolism | Stroke/systemic embolism | Stroke/systemic embolism | Stroke/systemic embolism |
| Primary safety endpoint | Major bleeding | Relevant bleeding | Major bleeding | Major bleeding |
| Contraindications | Hypersensitivity to the active substance/excipients Patients with severe kidney failure (CrCl <30ml/min) Clinically significant active bleeding Injury or disease, if it poses a significant risk of major bleeding Concomitant treatment with any other anticoagulant Liver failure or disease that may affect survival Concomitant treatment with systemic ketoconazole, cyclosporine, itraconazole and dronedarone Prosthetic heart valves require anticoagulant treat | Hypersensitivity to the active substance/excipients Clinically significant active bleeding Injury or disease, if it poses a significant risk of major bleeding Concomitant treatment with any other anticoagulant Liver disease associated with coagulopathy and clinically relevant bleeding risk, including cirrhotic patients with Child-Pugh B and C Pregnancy and breast-feeding | Hypersensitivity to active substance/excipients Clinically significant active bleeding Liver disease, associated with coagulopathy and clinically relevant bleeding risk Injury/disease poses a significant risk of major bleeding Concomitant treatment with any other anticoagulant agent | Hypersensitivity to the active substance/excipients Clinically significant active bleeding Liver disease, associated with coagulopathy and clinically relevant bleeding risk Injury or disease, if it poses a significant risk of major bleeding Uncontrolled severe hypertension Concomitant treatment with any other anticoagulant Pregnancy and breast-feeding |
Main efficacy and safety results of phase III clinical trials on embolism prevention in patients with non-valvular atrial fibrillation.
| RE-LY | ROCKET AF | ARISTOTLE | AVERROES | ENGAGEAF-TIMI 48 | ||
|---|---|---|---|---|---|---|
| D110 vs. warfarin % patients/year Relative risk (95% CI) | D150 vs. warfarin % patients/year Relative risk (95% CI) | Rivaroxaban vs. warfarin % patients/year Hazard ratio (95% CI) | Apixaban vs. warfarin % patients/year Hazard ratio (95% CI) | Apixaban vs. ASA % patients/year Hazard ratio (95% CI) | Edoxaban 60mg/30mg vs. warfarin % patients/year Hazard ratio (95% CI) | |
| Stroke or systemic embolism | 1.11 vs. 1.69%/year 0.91 (0.74–1.11) p<0.001 | 1.53 vs. 1.69%/year 0.66 (0.53–0.82) p=0.41 | 1.7 vs. 2.2%/year 0.79 (0.66–0.96) p<0.001a Intention-to-treat: 2.1 vs. 2.4%/year 0.88 (0.75–1.03) p<0.001b | 1.27 vs. 1.6%/year 0.79 (0.66–0.95) p=0.01 | 1.6 vs. 3.7%/year 0.45 (0.32–0.62) p<0.001 | 1.2 vs. 1.5%/year 0.79 (0.63–0.99) p<0.001 1.6 vs. 1.5%/year 1.07 (0.87–1.31) p<0.005 |
| Haemorrhagic stroke | 0.12 vs. 0.38%/year 0.31 (0.17–0.56) p<0.001 | 0.10 vs. 0.38%/year 0.26 (0.14–0.49) p<0.001 | – | 0.24 vs. 0.47%/year 0.51 (0.35–0.75) p<0.001 | 0.2 vs. 0.3%/year 0.67 (0.24–1.88) p=0.45 | 0.26 vs. 0.47%/year 0.54 (0.38–0.77) p<0.001 0.16 vs. 0.47%/year 0.33 (0.22–0.50) p<0.001 |
| Ischaemic stroke/not specified | 1.34 vs. 1.20%/year 1.11 (0.89–1.40) p=0.35 | 0.92 vs. 1.20%/year 0.76 (0.60–0.98) p=0.03 | – | 0.97 vs. 1.05%/year 0.92 (0.74–1.13) p=0.42 | 1.1 vs. 3.0%/yearc 0.37 (0.25–0.55) p<0.001 0.3 vs. 0.1%/yeard 2.24 (0.69–7.27) p=0.18 | 0.87 vs. 0.93%/year 0.94 (0.75–1.19) p<0.001 |
| Major bleeding | 2.71 vs. 3.36%/year 0.80 (0.69–0.93) p=0.003 | 3.11 vs. 3.36%/year 0.93 (0.81–1.07) p=0.31 | 14.9 vs. 14.5%/year 1.03 (0.96–1.11) p=0.44 | 2.13 vs. 3.09%/year 0.69 (0.60–0.80) p<0.001 | 1.4 vs. 1.2%/year 1.13 (0.74–1.75) p=0.57 | 2.75 vs. 3.43%/year 0.80 (0.71–0.91) p<0.001 1.61 vs. 3.43%/year 0.47 (0.41–0.55) p<0.001 |
| Intracranial haemorrhage | 0.23 vs. 0.74%/year 0.31 (0.20–0.47) p<0.001 | 0.30 vs. 0.74%/year 0.40 (0.27–0.60) p<0.001 | 0.49 vs. 0.74%/year 0.67 (0.47–0.93) p=0.019 | 0.33 vs. 0.80%/year 0.42 (0.30–0.58) p<0.001 | 0.4 vs. 0.4%/year 0.85 (0.38–1.90) p=0.69 | 0.39 vs. 0.85%/year 0.47 (0.34–0.63) p<0.001 0.26 vs. 0.85%/year 0.30 (0.21–0.43) p<0.001 |
| Clinically relevant bleeding | – | – | 11.80 vs. 11.37%/year 1.04 (0.96–1.13) p=0.345 | 4.07 vs. 6.01%/year 0.68 (0.61–0.75) p<0.001) | 3.1 vs. 2.7%/year 1.15 (0.86–1.54) p=0.35 | 11.1 vs. 13.0%/year 0.86 (0.80–0.92) p<0.001 8.0 vs. 13.0%/year 0.62 (0.57–0.67) p<0.001 |
Several post-marketing studies on the efficacy and safety of NOACs in clinical practice have been published, which in most cases confirm the conclusions from clinical studies on NOACs.
A systematic review and meta-analysis by Miller et al.23 concludes that as compared with warfarin, the NOACs (dabigatran, rivaroxaban, apixaban) reduce the risk of all-cause stroke and systemic embolism, ischaemic and unidentified cerebrovascular event, haemorrhagic cerebrovascular accident, all-cause mortality and vascular mortality. NOACs were also associated with a lower risk of intracranial bleeding. However, the data on serious bleeding and gastrointestinal bleeding were inconclusive.
A meta-analysis of the 4 main trials by Ruff et al.24 found that NOACs, compared with warfarin, significantly reduced the rate of cerebrovascular accident and systemic embolism. However, with the exception of apixaban, NOACs were associated with an increased risk of gastrointestinal bleeding. Another recent meta-analysis determined (except for D110 and edoxaban 30mg) that NOACs were superior against VKAs in the primary efficacy measure for reducing stroke/embolism; apixaban and edoxaban showed fewer bleeding events than D150, rivaroxaban and VKA. However, D110 and D150 were associated with a higher incidence of myocardial infarction as compared with apixaban, edoxaban 60mg and rivaroxaban.25 Although some other studies have found an association of dabigatran with an increased risk of myocardial infarction26: The Food and Drug Administration (FDA), after analysing data from 134,000 Medicare patients, concluded that in actual practice the incidence of myocardial infarction was similar with dabigatran and warfarin.27
In subgroup analyses, there are no references to the use of NOACs in children or, of course, in pregnant women or during lactation. A gender-based meta-analysis by Pancholy et al.28 suggests a net clinical benefit of NOACs as compared with warfarin in the treatment of women with non-valvular atrial fibrillation (NVAS). Other studies on groups of patients with or without previous stroke/transient ischaemic attack (TIA) or symptomatic heart failure have verified the superiority of D150 in relation to the primary efficacy measures for reduction of stroke and systemic embolism, whereas D110, apixaban and rivaroxaban were non-inferior as compared with warfarin, which was consistent with the results from the previous main studies.29–31
Criteria for anticoagulation in patients with atrial fibrillationAn attempt to restore sinus rhythm, if indicated, or to control heart rate in patients with persistent or permanent AF, should be accompanied by strategies to prevent embolic events.
The classic anticoagulants are the VKAs. Anticoagulant treatment with VKAs reduces the risk of stroke by 67% and all-cause mortality by 26%.32 However, their pharmacokinetic and pharmacodynamic profiles are significantly modified not only by many drug interactions and genetic polymorphisms, but also by dietary vitamin K intake, which requires continuous clinical monitorisation to maintain the international normalised ratio (INR) in the therapeutic range between 2 and 3.33
The period of time within the therapeutic range affects the results of the drug; being in therapeutic range 70% of the time reduces the risk of stroke by 5 fold as compared with those who are only a 30% of the time. The risk of bleeding, death, and myocardial infarction also decreases.34
Two recent Cochrane reviews studied the effects of oral anticoagulants on stroke prevention. In patients with no prior history of stroke,35 treatment with acenocoumarol with an INR of 2–3 reduces the number episodes of stroke and death in patients with non-valvular AF. In patients with a history of stroke, oral anticoagulants were associated with a significant increase in the risk of severe extracranial bleeding.36 The American Heart Association's (AHA)13 2014 clinical practice guidelines recommend oral anticoagulants in patients with non-valvular AF with a previous stroke, transient ischaemic episode or CHA2DS2-VASc score ≥2: warfarin with INR 2–3 (evidence A), dabigatran (evidence B), rivaroxaban (evidence B) or apixaban (evidence B).
Criteria for anticoagulation in patients with atrial fibrillation and advanced chronic kidney disease. ControversyOne of the problems to assess the efficacy of anticoagulants in CKD patients is that those with advanced stages of CKD have traditionally been excluded from the main trials due to the large amount of renal excretion (dabigatran, 80%; rivaroxaban, 1/3 of the unchanged active molecule is excrete in the urine and 2/3 of the rest also undergoes renal excretion, apixaban and edoxaban is excreted in the urine in a 25% and 35% respectively).
The number of patients with stage 4 CKD included in the RE-LY and ROCKET-AF studies with dabigatran and rivaroxaban, is small so it does not allow to draw conclusions. Notwithstanding, the FDA has approved a lower dose of dabigatran of 75mg twice daily based exclusively on pharmacokinetic and pharmacodynamic data, and some agencies have approved rivaroxaban at a dose of 15mg in this CKD population. Lower doses of apixaban (2.5mg/12h) and edoxaban (30mg/24h) are recommended. We might add, however, that there are no relevant data in this regard with VKAs in stage 4 CKD either, despite being recommended for AF by the Canadian guidelines.
Regarding CKD stage 5 patients, the AHA guidelines state the following terms:
- •
In patients with non-valvular AF and CHA2DS2-VASc score ≥2 with creatinine clearance (CrCl) <15ml/min) or on haemodialysis, anticoagulation with warfarin (INR 2.0–3.0) (evidence B) is recommended.
- •
New oral anticoagulants are not recommended in patients with CrCl <15ml/min or on haemodialysis in the absence of evidence in clinical trials of a risk/benefit balance (Table 6).
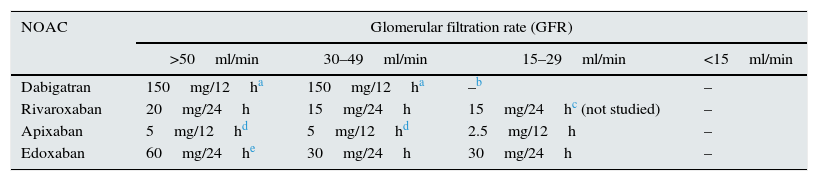
Table 6.Recommended doses of new oral anticoagulants (NOACs) according to glomerular filtration rates.
NOAC Glomerular filtration rate (GFR) >50ml/min 30–49ml/min 15–29ml/min <15ml/min Dabigatran 150mg/12ha 150mg/12ha –b – Rivaroxaban 20mg/24h 15mg/24h 15mg/24hc (not studied) – Apixaban 5mg/12hd 5mg/12hd 2.5mg/12h – Edoxaban 60mg/24he 30mg/24h 30mg/24h – aA dose reduction to 220mg (110mg/12h) should be considered in patients with a high risk of bleeding.
bThe FDA authorises a dose of 75mg/12h for patients with CrCl 15–30ml/min, based on pharmacokinetic models. The other regulatory agencies contraindicate dabigatran if CrCl is <30ml/min.
However, indicating warfarin in patients with stage 5 CKD (GFR <15ml/min or dialysis) is controversial. Olesen et al.37 conclude in favour of anticoagulation with acenocoumarol. In patients with AF, CKD increases the risk of stroke, thromboembolism and bleeding, and anticoagulation with warfarin, as in patients without CKD, reduces the risk of stroke and thromboembolism, thereby increasing the risk of bleeding. ASA increases the risk of bleeding but does not reduce episodes of stroke or thromboembolism.
There are 3 publications including extensive registries that concluded that anticoagulation with warfarin in patients with AF on haemodialysis was associated with an increased risk of stroke.38–40 In addition, in haemodialysis patients on warfarin, the risks of bleeding, vascular calcification and calciphylaxis are increased.41
Consequently, the use of acenocoumarol to prevent thromboembolic events in dialysis patients with non-valvular AF is discouraged. In fact, due to lack of evidence, the European Society of Cardiology states the following: “CKD may increase the risk of thromboembolism in AF, although these patients also have an increased risk of mortality and bleeding but there are no prospective studies to draw definite conclusion”.14
Comparison of new oral anticoagulants. Selection criteriaTo date, no studies have been published showing a direct comparison between these 4 NOACs. Thus, It is difficult to establish any superiority of any of them. A study by Schneeweiss et al.,42 in which an indirect comparison was made between dabigatran, rivaroxaban and apixaban, indicated that in patients with CHADS2 ≥3, D150mg, apixaban 5mg and rivaroxaban 20mg were similar in rates of stroke and systemic embolism, but apixaban had a lower risk of major bleeding as compared with dabigatran and rivaroxaban. Another recent meta-analysis by Fu et al.43 makes indirect comparisons concerning the safety and efficacy of these 4 NOACs. Compared with D150, rivaroxaban and edoxaban 30mg showed a significantly higher risk of stroke and systemic embolism and, rivaroxaban showed a higher risk of haemorrhagic stroke. There was an increased risk of stroke or systemic embolism, transient ischaemic attack (TIA), ischaemic stroke and incapacitating or fatal TIA with edoxaban 30mg as compared with apixaban and rivaroxaban with the exception of rivaroxaban and the incidence of stroke or systemic embolism. Apixaban, rivaroxaban, and edoxaban 60mg may significantly reduce the risk of myocardial infarction compared with D150. Regarding the safety, the risks of major bleeding, gastrointestinal bleeding and any other type of bleeding were significantly higher with rivaroxaban than with apixaban and D110; as edoxaban 60mg vs edoxaban 30mg. Edoxaban 30mg showed a significant reduction in major bleeding compared with the other NOACs, and vs D150, D110 and rivaroxaban.
With no direct comparative studies among these NOACs, the selection of one or the other must depend on the physician's view of the benefit in each study. The specific recommendations accepted by the regulatory agencies and, above all, the (necessary) economic constraints imposed by the different funding agencies, including patients’ purchasing power. A recent systematic review of 23 studies by Ferreira and Mirco44 concluded that NOACs are beneficial in preventing stroke in AF, and, without analysing edoxaban, given its very recent approval, it seems that apixaban is the most rewarding, followed by dabigatran and rivaroxaban. However, given the limitations of the different analytical models used, and until further studies are performed, these results should be considered with caution.
Key concepts- •
AF is the most common chronic cardiac arrhythmia, and its prevalence in CKD is 10–20 times higher than in the general population, with the frequency of ischaemic stroke increasing as renal function declines.
- •
VKAs reduce the risk of stroke and all-cause mortality. However, their pharmacokinetic and pharmacodynamic profiles are significantly affected by many drug interactions, genetic polymorphisms, as well as by dietary vitamin K intake.
- •
NOACs, in addition to showing a wide therapeutic range, have little variability, a rapid onset of action and a short half-life. Moreover, they can be given in fixed doses without the need for monitoring, owing to their pharmacokinetic and pharmacodynamic properties, they have few interactions and show a variable degree of renal excretion.
- •
There is now a direct thrombin inhibitor (dabigatran) and 3 direct inhibitors of activated factor X (rivaroxaban, apixaban and edoxaban), with approved indications for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF).
- •
For GFR between 30 and 50ml/min, the doses of dabigatran and rivaroxaban should be reduced (if there is a high risk of bleeding), and it is not necessary to reduce the doses of apixaban and edoxaban. For GFR from 15 to 29ml/min, dabigatran is contraindicated, dose of rivaroxaban and edoxaban do not need to be adjusted, while apixaban does. None of the NOACs is indicated for GFR below 15ml/min.
- •
There is currently a specific reversal agent for dabigatran, indicated in patients requiring surgery or with severe bleeding. For the rest of the NOACs, new specific reversal agents are expected soon.
- •
NOACs are more expensive than VKAs. However, their cost tends to decrease as new drugs come on the market. However, the lower risk of thromboembolic events and bleeding provides a cost-effective alternative compared with VKAs.
The authors declare that there are no conflicts of interest.
Please cite this article as: Belmar Vega L, de Francisco ALM, Bada da Silva J, Galván Espinoza L, Fernández Fresnedo G. Nuevos anticoagulantes orales en pacientes con enfermedad renal crónica. Nefrologia. 2017;37:244–252.