Inflammation is one of the central pathophysiological factors in kidney disease. Nowadays, we have widely recognised diagnostic and monitoring markers, such as C-reactive protein, serum albumin, erythrocyte sedimentation rate, ferritin, tumour necrosis factor, apolipoprotein A-1, interleukin-1, interleukin-6 and many others.1
However, in the present socioeconomic status, it is important that we seek cost-effective biological markers. After extrapolating their utility from other areas, the platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) have begun to be used in kidney patients, particularly as markers of inflammation, endothelial damage and, more recently, as predictor of death.1–3
The purpose of this review was to perform an in-depth analysis of the pathophysiological bases of these indexes, with special emphasis on the processes of inflammation and arteriosclerosis, and their clinical utility in kidney patients.
Pathophysiological bases of the ratiosNeutrophilsIn classical terms, neutrophils are immature phagocytes with a short half-life. They are known to have the ability to release proteolytic enzymes and oxygen free radicals, actively contributing to the damage produced during inflammatory processes.
In vascular territory, neutrophils interact with the endothelium, releasing various proteins from their granules that generate molecular instructions to recruit and activate other inflammatory cells. Leukotrienes, which also form part of the arsenal of neutrophils, characteristically have significant capacity to evoke chemotaxis. All these actions amplify the initial process and trigger a significant immunoregulatory effect.4
However, classically, the role of neutrophils in atherosclerosis was not given much importance in view of their relative absence in conventional histological studies due either to their short half-life or their phenotypic evolution to other cells (for example, macrophages or dendritic cells, etc.).4
That situation changed with the advent of new immunological markers (such as Ly6G, anti-MPO and CD66b), as neutrophils have now been detected in early and advanced atherosclerosis lesions, in subendothelial and intimal locations and even inside thrombi.4–6
Another property of neutrophils is their ability to produce extracellular tissue scaffolds (Neutrophil Extracellular Traps), which are designed to trap pathogens. Interestingly, however, these supramolecular complexes have also been implicated in the atherosclerosis process.7,8
In a hyperlipidaemic environment, activated neutrophils can worsen endothelial function, creating proinflammatory positive feedback and decreasing vasodilation. Added to that, the adhesion of neutrophils to the endothelium stimulates the release of secretory vesicles that perpetuate the endothelial dysfunction.4
The presence of activated neutrophils has also been associated with the destabilisation of atherosclerotic plaques through oxygen free radicals and some extracellular matrix proteinases (matrix metalloproteinases [MMP]). In some experimental studies, treatment with fluvastatin decreased these MMP and increased collagen, a situation that produces negative feedback decreasing neutrophil infiltration.9
Therefore, it is now accepted that activated neutrophils are key elements in atherogenesis and subsequent cardiovascular risk.7,8
PlateletsPlatelets are nucleated cell fragments derived from megakaryocytes. Their haemostatic and prothrombotic function has been widely studied.10,11 However, their proinflammatory function has been investigated only recently.10 Platelets interact with numerous immune cells, but their relationship with endothelial cells and leucocytes are among the most important. This association has been fundamental in understanding the pathophysiology of vascular inflammation.10,12
In atherosclerosis, the presence of inflammation inhibits the antiadhesion properties of platelets, which tends to increase the interaction of platelets with the endothelium. This circumstance sets off a series of inflammatory effects in cascade, analogous to the phenomena that occur in thrombosis and haemostasis. Greater platelet activation triggers the secretion of cytokines and, in turn, creates a “chemotasis” effect that some authors have termed “inflamed endothelium”.12
There is also an important interaction between platelets and leucocytes in the context of atherogenesis, promoting cell recruitment to the area of the lesion through selectins and integrins.13,14 Specifically: connective tissue activator peptide III (CTAP-III) is transformed into neutrophil activating protein-2 (NAP-2), which in vitro induces neutrophil and monocyte adhesion as well as transendothelial migration of neutrophils.15–17
LymphocytesThe effects of lymphocytes on atherosclerosis can be both deleterious due to the effect of Th1 cells and protective due to the action of regulatory Th2 and CD4+Foxp3+cells (Tregs).18,19
The presence of oxidised LDL stimulates the activation of dendritic cells, which favours the balance of T cells towards a predominance of the “pro-atherogenic” line including Th1 or Th17.18,20
The Th2 lymphocytes, through interleukin-19 (IL-19), induce an anti-inflammatory state that favours pathways such as GATA3 and Foxp3. These pathways attenuate or decrease the atherosclerotic process. Perhaps this may be the reason why an increase in circulating Th2 is associated with a lower risk of myocardial infarction.18,21
The “good” Tregs cells are diminished in murine models of a hyperlipidaemic diet that leads to atherosclerosis. This diet would increase the chemotaxis of the lymphocytes towards the aorta and promotes the development and progression of aortic atherosclerosis.18,19,22
In relation to CD8 cells, opinions of different authors are divided; there are studies that consider that CD8 cells are pro-atherogenic, but a subgroup of these cells (CD8+ CD25+) would have the opposite effect.18
Other less common cell lines also significantly influence the atherosclerosis process. For example, γδ lymphocytes which produce interleukin-17 (IL-17) have been surprisingly found in the aortas of murine apoE -/- models fed a Western diet. NK (natural killer) cells, whose primary function is to confront viruses and other pathogens as part of the body's innate defence system, could contribute to atherosclerosis. It has been demonstrated that in murine models without NK cells the atherosclerosis process was attenuated. The involvement of NK cells in the rupture of the atherosclerotic plaque has also been described by some authors.23
The B lymphocytes also affect the atherosclerotic process. It is known that B1 cells protect against atherosclerosis. However, the role of B2 cells remains unclear.18
InflammationTaken together all the information available1,2 it is concluded that these mentioned indexes could be a marker of inflammatory imbalance in which there is a predominance of effector cells (pro-inflammatory effect) mainly activated neutrophils and platelets, over regulatory cells (anti-inflammatory effect), CD4 cells in particular. However, the model is complicated because, as previously mentioned, some lymphocyte cell subtypes have a dual effect. For example, a predominance of the subtype of T-helper 17 cells would result in increased production of IL-17 which is involved in atherogenic processes.24
First clinical uses of the ratiosThe first references to NLR are in relation to severe infections and changes in adrenocortical function in animals under stress.25–27 In later years, the Ventafrida group mentions the leucocyte-to-lymphocyte ratio as a predictor of survival in patients with cancer cachexia.28
In 1995, Goodman et al. published one of the first clinical uses of NLR in humans as a marker of acute appendicitis: an NLR ≥3.5 had a greater sensitivity for detecting the disease than the absolute number of leucocytes.29 A year later, it was used in conjunction with the ADA test for the diagnosis of pleural tuberculous.30
Its use as a prognostic marker in cancer continues to extend to gastric and colorectal disease,31,32 while also being applied in critically ill patients, comparing it with other widely recognised scales, such as APACHE II.33
From a cardiovascular point of view, in 2006 Duffy et al. published one of the first studies to link NLR levels prior to percutaneous coronary intervention with a higher subsequent mortality rate.34 In 2008, Papa et al. described the NLR as a predictor of cardiac death in patients with stable coronary heart disease; the patients at highest risk had an NLR above 2.55.35
The use of the PLR is more recent and was initially used as a marker in periampullary cancer.36,37 The first studies correlating the PLR with cardiovascular disease appeared in 2012.38
Use in oncologyWithin this field, inflammation has gained a great deal of interest both from the pathophysiological and prognosis point of view.39,40
Neutrophils inhibit the suppressive function of CD8 cells. That is why an increase in neutrophils would be associated with a worse response to cancer cells. In contrast, the presence of lymphocytes in some cancers has been associated with a better response to treatment.41
The NLR has specifically been described as a prognostic marker in different types of cancers such as breast, colon, kidney, urothelium and pancreas.39,42
The PLR has also been used as a good prognostic marker in colorectal, gastroesophageal, hepatic, pancreatic, ovarian and breast cancer.43
Use in cardiovascular diseaseIn previous sections, we have discussed the influence of neutrophils, platelets and lymphocytes on systemic inflammation and their relationship with the arteriosclerotic process. The arteriosclerotic process is associated with a greater long-term cardiovascular risk.24
In coronary heart disease, the NLR is associated with a higher degree of coronary obstruction, worse prognosis and a higher frequency of major cardiovascular events (acute myocardial infarction, revascularisation and death of cardiovascular cause).24,44 The NLR predicts death not only in stable patients but also in acute disease. In some studies, a high NLR value prior to a percutaneous intervention was associated with higher rates of stent restenosis45 and post-intervention mortality.46
In cerebrovascular disease, the NLR is a good predictor of death, functional dependence and severity of stenosis.24
In peripheral vascular disease, the NLR is associated with greater disease severity and high rates of both critical stenosis and amputation. If we consider treated patients, a high NLR is associated with a higher rate of amputation in the first month post-embolectomy and with graft failure in revascularisation surgery. In view of these findings, its association with higher mortality rates is not surprising, whether in patients receiving active treatment or those managed conservatively.24
In patients with diabetes, the NLR can also be a marker of major cardiovascular events, even more accurate than albuminuria.47 Other authors have even found a significant association between a high NLR values and HOMA index. This finding shows the probable association between inflammation and insulin resistance.48
Some studies have linked the NLR with endothelial dysfunction in clinically asymptomatic patients. Martínez-Urbistondo et al. found a good correlation between high NLR and an abnormal urinary albumin/creatinine ratio. Interestingly, in this same study, patients on treatment with statins had a tendency to have a lower NLR, probably due to the anti-inflammatory effect of these drugs.49
Use in kidney diseaseAcute kidney injuryNephrologists are eager to find a parameter capable to predict for acute kidney injury (AKI). In cardiac surgery, different markers such as cystatin C, interleukin-18, kidney injury molecule-1 (KIM-1), N-acetyl-beta-d-glucosaminidase and neutrophil gelatinase-associated lipocalin have been tested.3 However, their use is not widespread and is generally confined to the scope of clinical research. Some recent retrospective studies have shown their interest in the NLR as a marker of AKI in postoperative cardiac patients. A study of 590 patients showed that a high NLR in the postoperative period after cardiac surgery could be a predictor of AKI. The authors suggest that the intraoperative ischaemia and the use of an extracorporeal circuit may activate the renal endothelial cells and create a renal inflammatory state that could lead to the development of AKI. In this study, the one-year overall mortality rate was higher in quartiles 3 and 4 of the postoperative NLR.3
Kidney disease progressionSome studies have found a negative correlation between the NLR and the glomerular filtration rate. Tonyali et al. found that an NLR >3.18 in patients undergoing radical or partial nephrectomy was associated with an increased risk (almost 3 times) of developing CKD (defined by a glomerular filtration rate <60ml/min/1.73m2).50
In another recent study, Lu et al. reported that a high NLR was associated with a greater likelihood of renal replacement therapy.51
Vascular access stenosisNative or prosthetic arteriovenous fistulas are the most efficient types of vascular access in the field of haemodialysis and the main reason for dysfunction is stenosis. Stenosis occurs due to the development of venous intimal hyperplasia caused by what is initially damage to the endothelium, but which goes on to trigger a series of detrimental effects (oxidative stress, inflammation, endothelial dysfunction and migration of neointimal cells from alternative areas).52
Microscopicaly, venous intimal hyperplasia is similar to atherosclerosis.53 The mechanisms related to the stenosis of native and prosthetic fistulas could therefore be analogous to those described in coronary procedures, in which there is an inflammatory imbalance and markers such as the NLR are elevated.54 With this premise, several studies have suggested that the NLR could be a good marker of stenosis and re-stenosis of native and prosthetic arteriovenous fistulas.53,54 One of these studies reports an NLR cut-off point ≥2.7 for the determination of stenosis, with an ROC curve and AUC of 0.893, representing a sensitivity of 98.4% and a specificity of 75%.53 However, these are cross-sectional or retrospective studies with a low level of scientific evidence.53,54
Resistance to erythropoietin and inflammationIn the field of haemodialysis, patients with resistance to erythropoietin have, independently, higher morbidity and mortality rates. One of the factors that most influences resistance to erythropoietin is the patients’ own inflammatory state.55 In the last few years, studies have analysed the association between NLR and PLR, with resistance to erythropoietin based on inflammation as a common link.56
It has been suggested that the PLR could be a good marker of inflammation in the population with chronic kidney disease category G5. Recent publications consider that the PLR has a better predictive value for diagnosing inflammation than the NLR.1,2
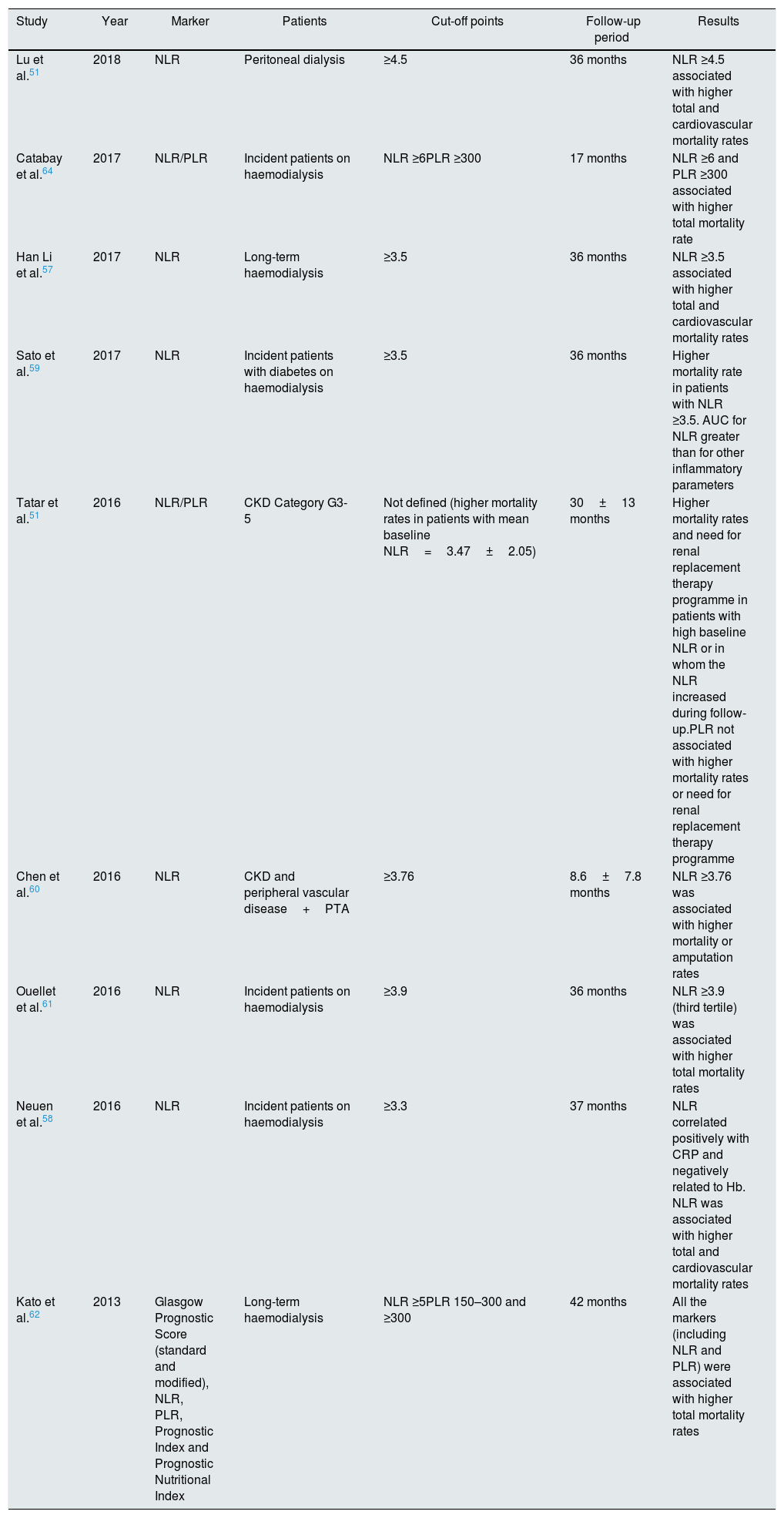
Mortality rates in patients with chronic kidney diseaseAlso in the last few years a number of studies have assessed the importance of the NLR and PLR as markers of death in patients with advanced chronic kidney disease, patients on haemodialysis and, more recently, those on peritoneal dialysis. A relationship has been found between high values for these ratios and higher total and cardiovascular mortality rates in renal patients (Table 1).51,57–63
Clinical studies of NLR/PLR as predictors of death in patients with chronic kidney disease.
| Study | Year | Marker | Patients | Cut-off points | Follow-up period | Results |
|---|---|---|---|---|---|---|
| Lu et al.51 | 2018 | NLR | Peritoneal dialysis | ≥4.5 | 36 months | NLR ≥4.5 associated with higher total and cardiovascular mortality rates |
| Catabay et al.64 | 2017 | NLR/PLR | Incident patients on haemodialysis | NLR ≥6PLR ≥300 | 17 months | NLR ≥6 and PLR ≥300 associated with higher total mortality rate |
| Han Li et al.57 | 2017 | NLR | Long-term haemodialysis | ≥3.5 | 36 months | NLR ≥3.5 associated with higher total and cardiovascular mortality rates |
| Sato et al.59 | 2017 | NLR | Incident patients with diabetes on haemodialysis | ≥3.5 | 36 months | Higher mortality rate in patients with NLR ≥3.5. AUC for NLR greater than for other inflammatory parameters |
| Tatar et al.51 | 2016 | NLR/PLR | CKD Category G3-5 | Not defined (higher mortality rates in patients with mean baseline NLR=3.47±2.05) | 30±13 months | Higher mortality rates and need for renal replacement therapy programme in patients with high baseline NLR or in whom the NLR increased during follow-up.PLR not associated with higher mortality rates or need for renal replacement therapy programme |
| Chen et al.60 | 2016 | NLR | CKD and peripheral vascular disease+PTA | ≥3.76 | 8.6±7.8 months | NLR ≥3.76 was associated with higher mortality or amputation rates |
| Ouellet et al.61 | 2016 | NLR | Incident patients on haemodialysis | ≥3.9 | 36 months | NLR ≥3.9 (third tertile) was associated with higher total mortality rates |
| Neuen et al.58 | 2016 | NLR | Incident patients on haemodialysis | ≥3.3 | 37 months | NLR correlated positively with CRP and negatively related to Hb. NLR was associated with higher total and cardiovascular mortality rates |
| Kato et al.62 | 2013 | Glasgow Prognostic Score (standard and modified), NLR, PLR, Prognostic Index and Prognostic Nutritional Index | Long-term haemodialysis | NLR ≥5PLR 150–300 and ≥300 | 42 months | All the markers (including NLR and PLR) were associated with higher total mortality rates |
It is suggested that both a high baseline NLR and increases in the NLR could be related to a greater requirement for renal replacement therapy and higher mortality rates.63 The cut-off point proposed by some authors from which the risk would be significantly elevated is around an NLR ≥3.5. The strong association between a high NLR and low levels of serum albumin strengthen the pathophysiological grounds for NLR as a marker of death. Furthermore, the greater predictive utility that NLR could provide over albumin is that its elevation in blood is faster (6–8h) than the decrease in albumin (19–21 days).64
In relation to the PLR, there are not enough data to define a definitive cut-off point. However, a recent study with over 100,000 incident patients on haemodialysis has established a J-curve pattern for death: values less than 100 and greater than 300 would have higher mortality rates than those in the range 100–150.57–62,64
Key concepts- •
Neutrophils participate actively in the atherosclerosis process, creating a proinflammatory environment through the Neutrophil Extracellular Traps.
- •
Activated neutrophils are involved in the destabilisation of atherosclerotic plaques.
- •
The interaction of platelets with the endothelium increases the migration of inflammatory cells to the area of the lesion by the use of platelet proteins.
- •
Th1 lymphocytes are pro-atherogenic, while Th2 and Treg cells are anti-atherogenic.
- •
NK cells are involved in the rupture of the atherosclerotic plaque.
- •
In oncological disease, a high NLR is described as a marker of poor prognosis in breast, colon, ovarian, kidney, urothelial and pancreatic cancers.
- •
In heart disease, the NLR is associated with a greater frequency of major cardiovascular events, a greater degree of coronary obstruction and a high rate of re-stenosis.
- •
In peripheral vascular disease, the NLR is associated with greater disease severity, a higher risk of amputation and graft failure in revascularisation surgery. It is also associated with a higher mortality rate.
- •
The NLR has been found to be a good marker of AKI in postoperative cardiac patients, of kidney disease progression and of a higher rate of admission in renal replacement therapy.
- •
The PLR is related to resistance to erythropoietin and could be a better inflammation marker than the NLR in the population with chronic kidney disease category G5.
- •
The NLR is a good marker of death in patients with CKD, with patients with an NLR ≥3.5 being at the highest risk.
- •
The PLR has a J-curve pattern with respect to death in incident patients on haemodialysis.
The authors have no conflicts of interest to declare.
Please cite this article as: Valga F, Monzón T, Henriquez F, Antón-Pérez G. Índices neutrófilo-linfocito y plaqueta-linfocito como marcadores biológicos de interés en la enfermedad renal. Nefrologia. 2019;39:243–249.