In recent years, monoclonal antibody therapy has increased exponentially, acquiring particular importance in patients with autoimmune diseases who are refractory to standard therapy. The use of these biological drugs has delivered great hope to this group of patients, as they have demonstrated promising results. The main drawback of these drugs is the current lack of knowledge on their possible side effects, due mainly to the fact that their use only started very recently and, therefore, experience with them in both the short- and long-term is limited.
An example of use of these treatments in the field of Dermatology is in psoriasis,1 where the most commonly used drugs are adalimumab (Humira®) and ustekinumab (Stelara®).2–4 The latter is an IgG1 kappa antibody, which acts through specific binding to the p40 subunit of interleukins 12 and 23, thereby preventing the interaction of these interleukins with their receptor. Through this mechanism, it is possible to block the activation pathway of natural killer cells and T cells, as well as to prevent the differentiation of CD4+ T cells from Th1 cells (helper cells).5,6
After reviewing the literature, we did not find any relationship between this treatment and nephrotic syndrome. We therefore consider this review interesting.
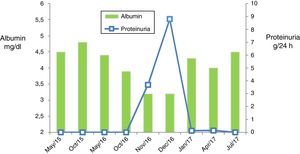
We present the case of a 51-year-old male with dyslipidaemia, undergoing treatment with statins and with a history of renal lithiasis of the left kidney during his youth, and undergoing follow-up by Dermatology for plaque psoriasis predominantly affecting the upper limbs, trunk and scalp. He had received treatment with Neotigason® (acitretin), topical corticosteroids, vitamin D analogues and ultraviolet A radiation, presenting a good response to treatment, but with re-occurrence of the lesions after its withdrawal. In this context, therapy was started with adalimumab, resulting in a partial response. In view of the persistent scalp and trunk lesions, it was decided to change to ustekinumab. This drug was administered in accordance with the normal induction regimen of 45mg, followed by another 45mg after four weeks, subsequently administering the next doses every 12 weeks.7 Approximately two years after starting treatment, when he had received a total dose of 585mg, a few days after administration of the last dose, the presence of proteinuria was observed in the sitematic urinary analysis that was confirmed in a 24-h urine sample. Initially the proteinuria was 3.7g/day and it was accompanied by mild hypoalbuminemia (albumin 3.2g/dl) with increased levels of cholesterol and triglycerides, maintaining preserved kidney function. He was therefore referred to Nephrology to test for possible glomerulopathy.
During one of our visits, the patient mentioned foamy urine, which he had been experiencing for a few weeks, without observing haematuria or other symptoms. Considering the possibility of glomerulonephritis it was tested for immunoglobulins, complement, with blood and urine electrophoresis, ANA, ANCA and viral serology. The results confirmed the presence of nephrotic syndrome. The remaining complementary tests were normal or negative. Finally, in view of the findings obtained, an ultrasound-guided percutaneous kidney biopsy was performed.
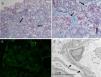
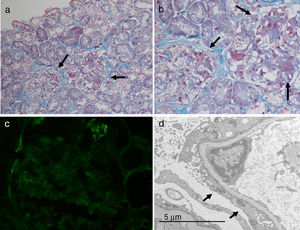
A total of 19 glomeruli were observed in the pathological study, none of which were completely sclerosed. The vast majority presented frequent synechiae between the tuft and Bowman's capsule. Discrete segmental consolidations were identified in three glomeruli. Focal segmental granular deposits of IgM and C3 were identified on the direct immunofluorescence study. Due to the above, he was diagnosed of focal segmental glomerulosclerosis classic type variant (Fig. 1).
Given the temporal relationship between the administration of the treatment and the onset of proteinuria, it was decided to discontinue ustekinumab in light of suspected side effects from treatment. Concomitant treatment with prednisone was also started at the standard treatment dose in accordance with the KDIGO guidelines used for the management of symptoms of primary glomerulosclerosis (mg/kg of weight).8 In this case, it was not possible to combine renin–angiotensin–aldosterone system inhibitors due to the patient's symptomatic hypotension. In the following visits, he presented favourable progress, testing negative for proteinuria and recovering from hypoalbuminemia from the first month of treatment initiation and discontinuation of ustekinumab. This rapid response, along with the absence of hypertension and microhaematuria, point to the diagnosis of side effects to treatment in the face of primary-origin glomerulonephritis. Despite this, it was decided to complete 12 weeks of oral prednisone, with progressive withdrawal until discontinuation. Currently, the patient continues to show no signs of active kidney disease (absence of proteinuria and normal kidney function) (Fig. 2).
This is a case with glomerular involvement in the form of focal segmental glomerulosclerosis demonstrated through microscopy studies. In accordance with the form of presentation and the rapid response after the discontinuation of ustekinumab, the symptoms could have been triggered by the treatment itself. This event is not mentioned in the drug's summary of product characteristics7 and so far it has not been reported in the literature. This may be due to the fact that it is an unknown side effect since, as mentioned beforehand, we still do not know the range of adverse effects that these drugs are capable of inducing as the use thereof has only started recently. In this case, it was considered a type B (idiosyncratic) serious adverse drug reaction, given that it directly affected the function of a vital organ such as the kidney.
We present a case report of a likely relationship between ustekinumab treatment and the onset of nephrotic syndrome secondary to focal segmental glomerulosclerosis, given the coincidence in time between them. The cause–effect relationship cannot be guaranteed, as it would be necessary to administer treatment with ustekinumab a second time and for proteinuria to reoccur. This procedure is not ethical and, therefore, it is not possible to demonstrate the cause–effect relationship in this case. However, in view of this fact, we recommend monitoring kidney function and proteinuria in patients who receive this drug.
Please cite this article as: Fernández MP, Piteiro Bermejo AB, Peña Esparragoza JK, Martínez AB, Moreno IA, Ramos JM, et al. Síndrome nefrótico en relación con tratamiento con ustekinumab. Nefrologia. 2019;39:100–102.