Chronic kidney disease patients have a high prevalence of vitamin D insufficiency/deficiency. Vitamin D deficiency has been associated with a variety of bone, metabolic and cardiovascular disorders. However, the role of native vitamin D supplementation (ergocalciferol, cholecalciferol or calcifediol) remains unclear in chronic kidney disease (CKD), particularly in the pre-dialytic phase. Several international guidelines have been developed on CKD–Mineral and Bone Disorder, but the optimal strategy for native vitamin D supplementation and its clinical benefit remains a subject of debate in the scientific community. This paper aims to review the available literature, including randomized clinical trials that evaluated the effects of native vitamin D supplementation on pre-dialysis CKD on biochemical and clinically relevant outcomes.
Los pacientes renales crónicos tienen una elevada prevalencia de insuficiencia/deficiencia de vitamina D. El déficit de vitamina D se ha asociado con una serie de cambios óseos, metabólicos y cardiovasculares. Sin embargo, continúa por aclarar el papel de la suplementación con vitamina D nativa (ergocalciferol, colecalciferol o calcifediol) en la enfermedad renal crónica (ERC), especialmente en la fase pre-dialítica. Varias pautas internacionales se han desarrollado sobre la enfermedad mineral y ósea relacionada con la ERC, pero la estrategia ideal de suplementación con vitamina D nativa y su beneficio clínico continúan siendo objeto de debate en la comunidad científica. Este trabajo pretende revisar la literatura disponible, incluyendo ensayos clínicos aleatorizados que evaluaron los efectos de la suplementación con vitamina D nativa en la ERC pre-diálisis en resultados bioquímicos y clínicamente relevantes.
Chronic kidney disease (CKD) is a relevant public health problem worldwide. In the United States of America population, prevalence of CKD patients over 30 years old reached 13.2% and is estimated to increase to 14.4% in 2020 and to 16.7% in 2030.1
Vitamin D deficiency is very frequent in CKD, affecting more than 80% of patients in pre-dialysis.2 Vitamin D insufficiency arises at an early stage of the disease and tends to worsen with the progressive loss of renal function.
Although mechanisms responsible for vitamin D deficiency are not fully understood, evidence suggests a strong inverse association between serum vitamin D concentration and morbidity and mortality in this population.3–5 Vitamin D has a pivotal role in mineral and bone metabolism regulation and acts on the cardiovascular and immune systems by the so-called pleiotropic effects. Vitamin D deficiency has been associated with increased risk of mortality, secondary hyperparathyroidism (SHPT), as well as increased cardiovascular risk, high blood pressure, diabetes, neoplasic and autoimmune diseases. Native vitamin D supplementation has been proposed as a strategy to minimize these consequences.6
The Kidney Disease: Improving Global Outcomes (KDIGO) group recommends vitamin D supplementation, but do not specify which agents and what is the optimal strategy to restore vitamin D levels.7 Native vitamin D supplementation (ergocalciferol, cholecalciferol and calcifediol) has been described in literature as an alternative to use of calcitriol, however, the effect of this supplementation on clinically relevant outcomes remains unclarified.8
Thus, this study aims to review the available literature regarding effects of native vitamin D supplementation in pre-dialysis chronic renal patients on biochemical (calcium, phosphorus, 25(OH)D, calcitriol, parathormone) and relevant clinical outcomes (hospitalizations, cardiovascular events and mortality). In addition, this paper will briefly review vitamin D metabolism, its deficiency in CKD and different regimens of supplementation.
Vitamin D metabolismVitamin D has a complex metabolism, which can be acquired by food or processed endogenously in the skin from sun exposure.5,9–12
Diet derived vitamin D includes ergocalciferol (D2) or cholecalciferol (D3) forms, but it constitutes a limited source since it can only be found in vegetables, yeasts and fish oils. Cutaneous synthesis results from a non-enzymatic process, through which absorption of UVB solar radiation (290–315nm) converts 7-dehydrocholesterol to previtamin D3. Then, previtamin D is converted into cholecalciferol by thermal isomerization.5,12,13
Vitamin D, either from diet or endogenously synthesized, is transported bound to vitamin D binding protein to the liver, where it is hydroxylated into 25-hydroxyvitamin-D (25(OH)D or calcidiol), by 25-hydroxylase enzyme. In turn, 25(OH)D is transported to the kidney, where it is converted into its active form, calcitriol (1,25-dihydroxyvitamin-D or 1,25(OH)2D) by the enzyme 1-α-hydroxylase.5,12–14 Renal hydroxylation, in contrast to hepatic hydroxylation, is highly regulated by serum levels of phosphorus, calcium, parathormone (PTH), FGF-23 and by active vitamin D itself.6 Similarly, 24-hydroxylase, the enzyme responsible for the calcitriol degradation, is also tightly controlled by serum levels of FGF-23, calcitriol itself and PTH.6,9,10,15
Calcitriol is responsible for vitamin D functions on mineral and bone metabolism, but it also has a pleiotropic effect on the modulation of endothelial function, immune response and regulation of the cell cycle.13,14,16,17 This vitamin increases plasma concentration of calcium and phosphorus, by stimulating intestinal absorption but also renal and bone resorption. The resulting hypercalcemia inhibits the secretion of PTH, which is also directly inhibited by vitamin D itself.15
Beyond renal expression, 1-α-hydroxylase enzyme is also present in other cells such as parathyroid cells, macrophages, osteoblasts, smooth muscle cells, endothelial cells and tissues such as the pancreas, breast, prostate and colon.5,6,8,9,18 Peripheral hydroxylation plays a key role, due to the possibility of converting 25(OH)D into its active form by maintaining adequate levels of 1-α-hydroxylase substract.8 This is the rationale for native vitamin D supplementation even in patients with impaired renal function.
Vitamin D metabolism disorders in chronic kidney diseaseVitamin D deficiency has a high prevalence in CKD and it is likely to worsen with kidney disease progression.9,15,19 In fact, Caravaca-Fontán et al. demonstrated that more than 80% of 367 pre-dialysis patients with a mean glomerular filtration rate (GFR) of 14.9±5.1mL/min/1.73m2, had plasma 25(OH)D concentration <20ng/mL.2
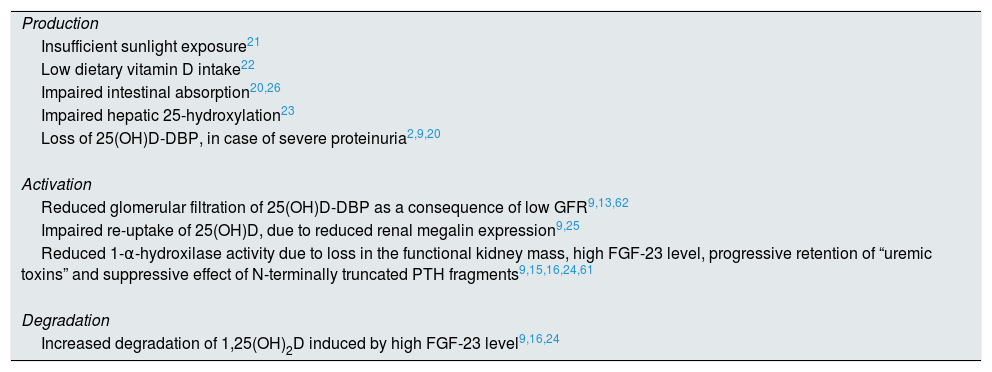
Several factors contribute to vitamin D deficiency in these patients, interfering with all phases of their metabolism, respectively, production, activation and degradation.2,6,9,13,15,16,19–26Table 1 summarizes the possible causes of deficiency, according to the respective phase of metabolism.
Factors contributing to impaired vitamin D metabolism.
| Production |
| Insufficient sunlight exposure21 |
| Low dietary vitamin D intake22 |
| Impaired intestinal absorption20,26 |
| Impaired hepatic 25-hydroxylation23 |
| Loss of 25(OH)D-DBP, in case of severe proteinuria2,9,20 |
| Activation |
| Reduced glomerular filtration of 25(OH)D-DBP as a consequence of low GFR9,13,62 |
| Impaired re-uptake of 25(OH)D, due to reduced renal megalin expression9,25 |
| Reduced 1-α-hydroxilase activity due to loss in the functional kidney mass, high FGF-23 level, progressive retention of “uremic toxins” and suppressive effect of N-terminally truncated PTH fragments9,15,16,24,61 |
| Degradation |
| Increased degradation of 1,25(OH)2D induced by high FGF-23 level9,16,24 |
In addition to these factors, vitamin D concentration seems to still be influenced by body mass index. Figuiredo-Dias et al. reported higher storage of vitamin D in adipose tissue with increased fat mass, which is no longer available for hepatic 25-hydroxylation.27
Since the early stages of CKD, there is an increase in levels of FGF-23 and PTH in an attempt to correct the trend toward hyperphosphataemia and hypocalcemia.9,28
FGF-23 inhibits renal phosphorous reabsorption and reduces serum levels of calcitriol by inhibiting renal 1-α-hydroxylase enzyme and stimulating 24-hydroxylase enzyme, which is responsible for vitamin D catabolism. So, FGF-23 contributes to phosphorus balance in pre-dialysis CKD, but aggravates calcitriol deficiency.9,10,15,24,28
Thus, SHPT develops as an inadequate response to hyperphosphatemia, hypocalcemia and progressive decline in calcitriol levels. PTH increases renal calcium reabsorption and tubular phosphorus excretion, also stimulating calcitriol synthesis, although in CKD, this process is compromised by hyperphosphatemia and functional renal mass reduction.6,15
The incidence and severity of SHPT increases as renal function decreases and may significantly interfere with bone remodeling and mineralization, increasing the risk of fractures and cardiovascular events.7,15
Despite the expected calcitriol deficiency in CKD, there is evidence that even in terminal CKD, there is a capability to convert 25(OH)D to calcitriol, emphasizing the importance of extra-renal production of calcitriol.6,8,18,29 So, native vitamin D supplementation has a growing interest among these patients, since it ensures substrate for peripheral hydroxylation, in order to attenuate calcitriol deficiency.
Assessment of Vitamin D statusThe quantification of 25(OH)D is the best biomarker to evaluate the vitamin D reserve in the body,30 because it has a long half-life (approximately 3 weeks) and allows to evaluate simultaneously the sources of vitamin D, respectively, nutritional intake and cutaneous synthesis.14
Until now, there is no consensus regarding 25(OH)D reference values due to the lack of a standardized method for quantification and the fact that the optimal level of 25(OH)D is based on PTH stabilization.11
Concerning 25(OH)D measurement, there are different types of assays. Although the gold standard is high-performance liquid chromatography, this is not widely available, because it is an expensive and slow method and also requires expertise and special instrumentation. Different assays have been developed, but the most used in the majority of studies is Diasorin automated chemiluminescence assay, which is co-specific for 25(OH)D2 and 25(OH)D3, reporting a total of 25(OH)D concentration.14
Despite divergence in studies, most consider that, in general population, vitamin D insufficiency corresponds to a serum 25(OH)D concentration between 20 and 29ng/mL and a deficiency <20ng/mL.5,31 Holick concluded that the serum 25(OH)D level of approximately 30ng/mL corresponded to the point at which the rise in 25(OH)D concentration was no longer reflected in plasma PTH concentration reduction.
In turn, the Institute of Medicine, for general population, considered a plasma concentration of 25(OH)D <12ng/mL as a deficiency, 12–19ng/mL as a insufficiency and ≥20ng/mL as an adequate concentration, stating that there is no benefit in concentrations ≥30ng/mL.32
However, the question remains whether these reference values are applicable to CKD patients. Ennis et al. after analysis of 14,289 chronic kidney patients found that concentrations of 25(OH)D >42–48ng/mL were required to achieve stable PTH levels, thus concluding higher vitamin D concentration to CKD patients.33
Although there is evidence suggesting higher levels of vitamin D, the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines, published in 2003, recommend a plasma 25(OH)D concentration ≥30ng/mL to prevent SHPT and to decrease the incidence of fractures in patients with stage 3 and 4 CKD.34
However, the Kidney Disease Improvement Global Outcomes (KDIGO) guidelines, recently published in 2017, do not consider any reference value for 25(OH)D level, recommending their evaluation, when PTH levels are progressively increasing or persistently above upper normal, at stages of CKD above 3. This recommendation, although recent, represents a low level of evidence due to absence of high quality scientific studies.7
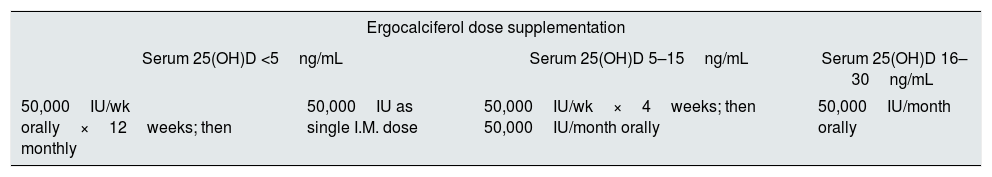
Vitamin D supplementation in CKDThe K/DOQI guidelines recommend correcting the plasma 25(OH)D concentration with ergocalciferol according to a 6-month regimen, as presented in Table 2.34 However, it is not yet clear in literature whether K/DOQI strategy is adequate to treat vitamin D deficiency and SHPT. Studies that tested the strategy suggested by K/DOQI did not find improvements in serum concentration of 25(OH)D or PTH.35,36
Ergocalciferol supplementation regimen for vitamin D insufficiency or deficiency in patients with CKD stage 3–4, according to K/DOQI.
| Ergocalciferol dose supplementation | |||
|---|---|---|---|
| Serum 25(OH)D <5ng/mL | Serum 25(OH)D 5–15ng/mL | Serum 25(OH)D 16–30ng/mL | |
| 50,000IU/wk orally×12weeks; then monthly | 50,000IU as single I.M. dose | 50,000IU/wk×4weeks; then 50,000IU/month orally | 50,000IU/month orally |
Later, KDIGO guidelines published in 2009, recently updated in 2017, recommend that deficiency and insufficiency of vitamin D in CKD with GFR <60mL/min/1.73m2 should be corrected through strategies provided to general population. According to the same guidelines, vitamin D supplementation in pre-dialysis patients should use native forms and reserve treatment with calcitriol and its analogs for more severe or progressive phases of SHPT.7
Native vitamin D supplementation has been of increasing interest in literature since there is evidence of 1-α-hydroxylase enzyme activity in extra-renal cells, giving the possibility of peripheral conversion of 25(OH)D to calcitriol.5,6,8,16
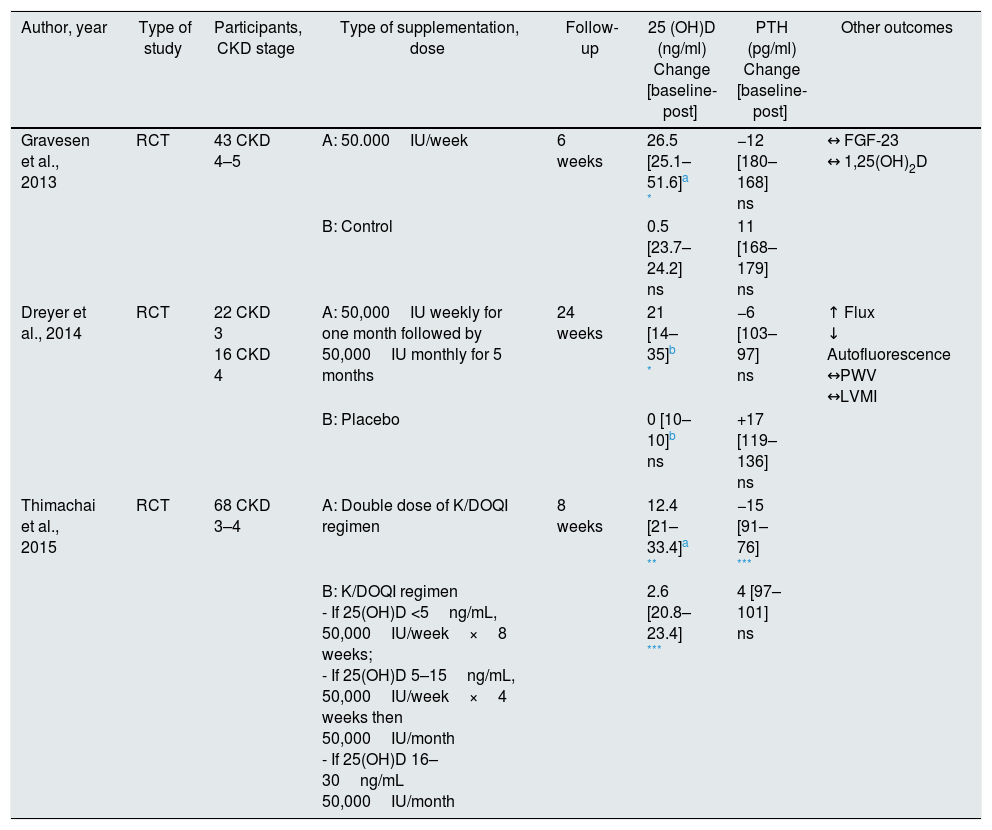
Native supplementation includes ergocalciferol or vitamin D2, cholecalciferol or vitamin D3 and calcifediol. There are many trials regarding the first two, being calcifediol the last agent to be studied. Tables 3–5 summarize the randomized controlled trials (RCT) that were performed with these three forms of native vitamin D for biochemical and clinically relevant endpoints.
Ergocalciferol supplementation in pre-dialysis CKD.
| Author, year | Type of study | Participants, CKD stage | Type of supplementation, dose | Follow-up | 25 (OH)D (ng/ml) Change [baseline-post] | PTH (pg/ml) Change [baseline-post] | Other outcomes |
|---|---|---|---|---|---|---|---|
| Gravesen et al., 2013 | RCT | 43 CKD 4–5 | A: 50.000IU/week | 6 weeks | 26.5 [25.1–51.6]a * | −12 [180–168] ns | ↔ FGF-23 ↔ 1,25(OH)2D |
| B: Control | 0.5 [23.7–24.2] ns | 11 [168–179] ns | |||||
| Dreyer et al., 2014 | RCT | 22 CKD 3 16 CKD 4 | A: 50,000IU weekly for one month followed by 50,000IU monthly for 5 months | 24 weeks | 21 [14–35]b * | −6 [103–97] ns | ↑ Flux ↓ Autofluorescence ↔PWV ↔LVMI |
| B: Placebo | 0 [10–10]b ns | +17 [119–136] ns | |||||
| Thimachai et al., 2015 | RCT | 68 CKD 3–4 | A: Double dose of K/DOQI regimen | 8 weeks | 12.4 [21–33.4]a ** | −15 [91–76] *** | |
| B: K/DOQI regimen - If 25(OH)D <5ng/mL, 50,000IU/week×8 weeks; - If 25(OH)D 5–15ng/mL, 50,000IU/week×4 weeks then 50,000IU/month - If 25(OH)D 16–30ng/mL 50,000IU/month | 2.6 [20.8–23.4] *** | 4 [97–101] ns |
Abbreviations: ns, not significant; 1,25(OH)2D, calcitriol; CKD, chronic kidney disease; FGF-23, fibroblast growth factor 23; K/DOQI, Kidney Disease Outcomes Quality Initiative; LVMI, left ventricular mass index; PWV, pulse wave velocity; PTH, parathyroid hormone; RCT, randomized controlled trial.
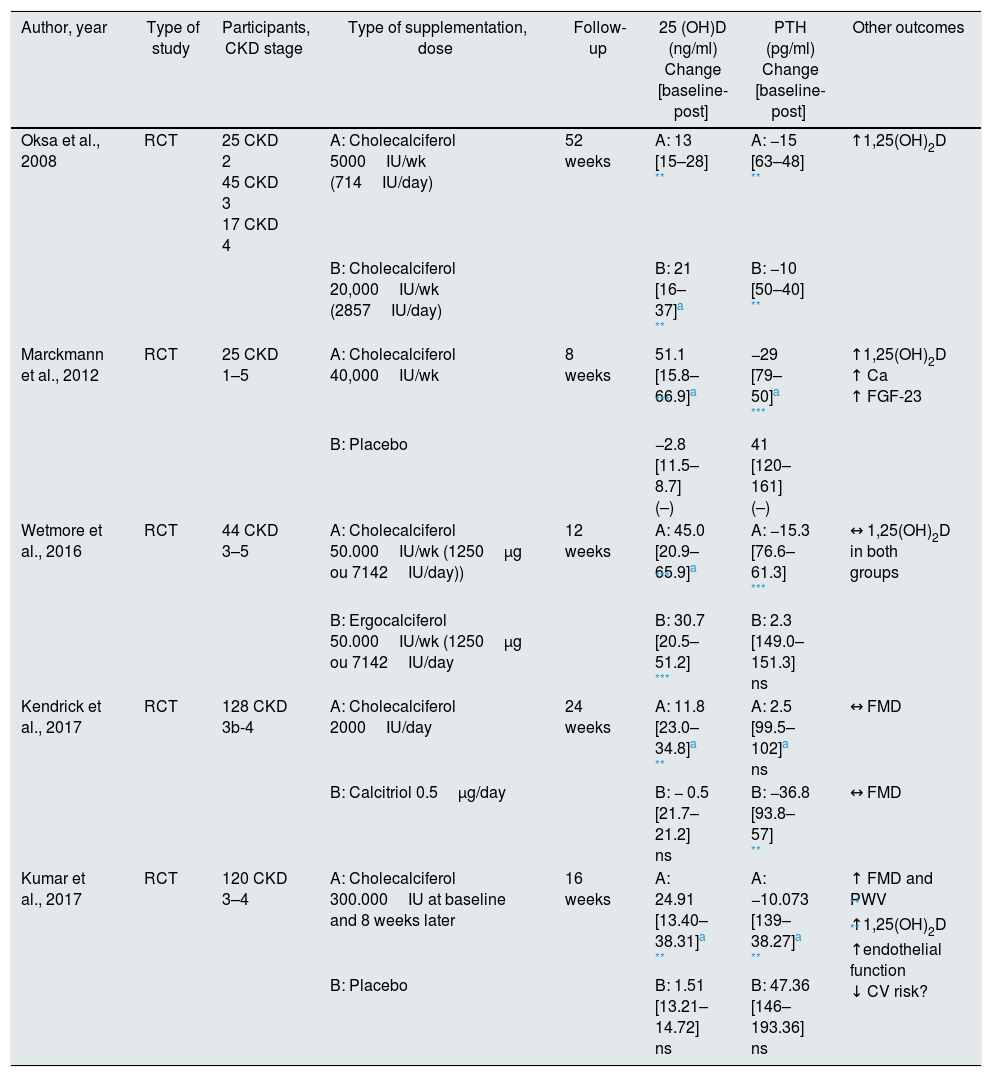
Cholecalciferol supplementation in pre-dialysis CKD.
| Author, year | Type of study | Participants, CKD stage | Type of supplementation, dose | Follow-up | 25 (OH)D (ng/ml) Change [baseline-post] | PTH (pg/ml) Change [baseline-post] | Other outcomes |
|---|---|---|---|---|---|---|---|
| Oksa et al., 2008 | RCT | 25 CKD 2 45 CKD 3 17 CKD 4 | A: Cholecalciferol 5000IU/wk (714IU/day) | 52 weeks | A: 13 [15–28] ** | A: −15 [63–48] ** | ↑1,25(OH)2D |
| B: Cholecalciferol 20,000IU/wk (2857IU/day) | B: 21 [16–37]a ** | B: −10 [50–40] ** | |||||
| Marckmann et al., 2012 | RCT | 25 CKD 1–5 | A: Cholecalciferol 40,000IU/wk | 8 weeks | 51.1 [15.8–66.9]a *** | −29 [79–50]a *** | ↑1,25(OH)2D ↑ Ca ↑ FGF-23 |
| B: Placebo | −2.8 [11.5–8.7] (–) | 41 [120–161] (–) | |||||
| Wetmore et al., 2016 | RCT | 44 CKD 3–5 | A: Cholecalciferol 50.000IU/wk (1250μg ou 7142IU/day)) | 12 weeks | A: 45.0 [20.9–65.9]a *** | A: −15.3 [76.6–61.3] *** | ↔ 1,25(OH)2D in both groups |
| B: Ergocalciferol 50.000IU/wk (1250μg ou 7142IU/day | B: 30.7 [20.5–51.2] *** | B: 2.3 [149.0–151.3] ns | |||||
| Kendrick et al., 2017 | RCT | 128 CKD 3b-4 | A: Cholecalciferol 2000IU/day | 24 weeks | A: 11.8 [23.0–34.8]a ** | A: 2.5 [99.5–102]a ns | ↔ FMD |
| B: Calcitriol 0.5μg/day | B: − 0.5 [21.7–21.2] ns | B: −36.8 [93.8–57] ** | ↔ FMD | ||||
| Kumar et al., 2017 | RCT | 120 CKD 3–4 | A: Cholecalciferol 300.000IU at baseline and 8 weeks later | 16 weeks | A: 24.91 [13.40–38.31]a ** | A: −10.073 [139–38.27]a ** | ↑ FMD and PWV ** ↑1,25(OH)2D ** ↑endothelial function ↓ CV risk? |
| B: Placebo | B: 1.51 [13.21–14.72] ns | B: 47.36 [146–193.36] ns |
Abbreviation: ns, not significant; 1,25(OH)2D, calcitriol; Ca, calcium; CKD, chronic kidney disease; CV, cardiovascular; FGF-23, fibroblast growth factor 23; FMD, flow mediated dilation; PWV, pulse wave velocity; PTH, parathyroid hormone; RCT, randomized controlled trial; (–), p-value not presented.
*p<0.0001.
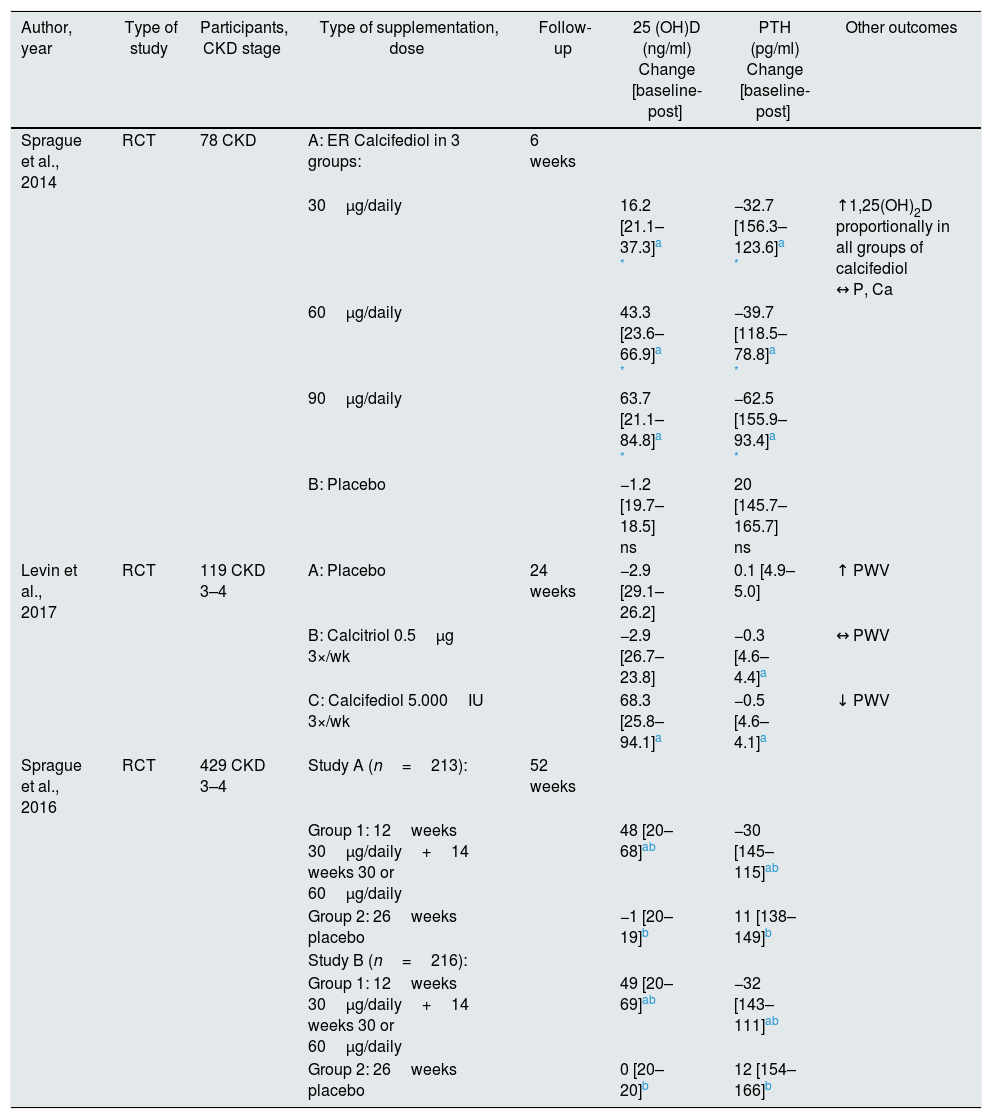
Calcifediol supplementation in predialysis CKD.
| Author, year | Type of study | Participants, CKD stage | Type of supplementation, dose | Follow-up | 25 (OH)D (ng/ml) Change [baseline-post] | PTH (pg/ml) Change [baseline-post] | Other outcomes |
|---|---|---|---|---|---|---|---|
| Sprague et al., 2014 | RCT | 78 CKD | A: ER Calcifediol in 3 groups: | 6 weeks | |||
| 30μg/daily | 16.2 [21.1–37.3]a * | −32.7 [156.3–123.6]a * | ↑1,25(OH)2D proportionally in all groups of calcifediol ↔ P, Ca | ||||
| 60μg/daily | 43.3 [23.6–66.9]a * | −39.7 [118.5–78.8]a * | |||||
| 90μg/daily | 63.7 [21.1–84.8]a * | −62.5 [155.9–93.4]a * | |||||
| B: Placebo | −1.2 [19.7–18.5] ns | 20 [145.7–165.7] ns | |||||
| Levin et al., 2017 | RCT | 119 CKD 3–4 | A: Placebo | 24 weeks | −2.9 [29.1–26.2] | 0.1 [4.9–5.0] | ↑ PWV |
| B: Calcitriol 0.5μg 3×/wk | −2.9 [26.7–23.8] | −0.3 [4.6–4.4]a | ↔ PWV | ||||
| C: Calcifediol 5.000IU 3×/wk | 68.3 [25.8–94.1]a | −0.5 [4.6–4.1]a | ↓ PWV | ||||
| Sprague et al., 2016 | RCT | 429 CKD 3–4 | Study A (n=213): | 52 weeks | |||
| Group 1: 12weeks 30μg/daily+14 weeks 30 or 60μg/daily | 48 [20–68]ab | −30 [145–115]ab | |||||
| Group 2: 26weeks placebo | −1 [20–19]b | 11 [138–149]b | |||||
| Study B (n=216): | |||||||
| Group 1: 12weeks 30μg/daily+14 weeks 30 or 60μg/daily | 49 [20–69]ab | −32 [143–111]ab | |||||
| Group 2: 26weeks placebo | 0 [20–20]b | 12 [154–166]b |
Abbreviations: ns, not significant; 1,25(OH)2D, calcitriol; Ca, calcium; CKD, chronic kidney disease; PWV, pulse wave velocity; P, phosphorus; PTH, parathyroid hormone; RCT, randomized controlled trial.
The Italian Nephrology Society published, in 2016, a “position statement” about vitamin D in CKD, suggesting to supplement patients in stage 3–5 CKD with a plasma 25(OH)D concentration <30ng/mL, using the scheme (ergocalciferol, cholecalciferol or calcifediol) with which the nephrologist is more confident.37
By contrast, the Spanish Society of Nephrology considered calcifediol as a more convenient option, although dosage and regimen to be used is not disclosed.38
It is important to emphasize that regardless of the native form chosen, the risk of toxicity should always be considered including adverse effects of hypervitaminosis D, such as hypercalcemia and hyperphosphataemia. Therefore, native vitamin D supplementation should be discontinued when serum 25(OH)D concentration is greater than 100ng/mL and/or when the serum calcium level exceeds 10.5mg/dl, in the absence of administration of calcitriol.37
Vitamin D supplementation and biochemical endpointsMost of studies indicate that vitamin D supplementation improves plasma 25(OH)D concentration, with different effects on calcitriol and PTH levels.
Reports show that ergocalciferol is less effective to the increase of 25(OH)D plasma concentration, due to its plant origin, lower half-life,39,40 a methyl group at C24 which confers lower conversion to 25(OH)D2 and also lower affinity to the binding protein41 by its faster catabolism.42
In addition, Armas et al. demonstrated in a randomized trial that the administration of a single oral dose of 50,000IU of ergocalciferol or cholecalciferol in 20 healthy men, 10 in each group, produced similar initial increases in plasma 25(OH)D concentration (mean plasma 25(OH)D concentration after 3 days, 27.5ng/mL in the ergocalciferol group vs. 29.9ng/mL in the cholecalciferol group, p>0.05) indicating equivalent absorption. However, after 4 weeks, cholecalciferol maintained high concentration of 25(OH)D for more time (mean change in plasma 25(OH)D concentration 33.7ng/mL), whereas in the ergocalciferol group, it had returned to the baseline level after 2 weeks. Comparing the area under the curve, cholecalciferol showed a more than 3-fold difference in potency.43
To evaluate the impact of ergocalciferol supplementation as recommended by K/DOQI, Qunibi et al. performed a retrospective observational study, which included 88 patients with 25(OH)D insufficiency or deficiency from all stages of CKD. The authors concluded that the dosage strategy recommended by K/DOQI is inadequate for vitamin D insufficiency or deficiency correction and treatment of SHPT. In fact, only 25% of treated patients reached the reference value of 30ng/mL.35
In a previous prospective observational paper, Zisman et al. used an adapted K/DOQI guideline scheme in 24 stage 3 patients and 28 stage 4 CKD patients. After an average period of 7 months, plasma 25(OH)D concentration increased from 20.3 to 31.6ng/mL (p<0.0001) in stage 3 patients and from 18.8 to 35.4ng/mL (p<0.0001) in stage 4 patients. In this study 58% and 68% of stage 3 and 4 patients, respectively, reached 30ng/mL in 25(OH)D level. This increase was associated with increased concentrations of calcitriol in both stage 3 and stage 4.44
About plasma PTH concentration, study by Qunibi et al. found no significant decrease35 and study by Zisman et al. found a significant decrease only in stage 3 patients (from 154.1 to 130.5pg/mL (p=0.041) with no relevant changes in stage 4 patients, which led the authors to conclude that ergocalciferol would be useful only for stage 3 patients with no benefit in stage 4 of CKD.44
Beyond the controversy over the efficiency of the proposed recommendations, several RCTs were conducted to better evaluate the effect of ergocalciferol supplementation. The RCT by Gravensen et al. enrolled 43 patients with stage 4 and 5 CKD, who underwent 50,000IU of ergocalciferol per week. The authors found an improvement in plasma 25(OH)D concentration (25.1±6.4 for 51.6±10.8ng/mL, p<0.0001), without significant improvement at PTH level. Since this study lasted 6 weeks, it could have been an insufficient period to promote changes in PTH.16 However, also in the 24-month RCT by Dreyer et al., it was only found plasma 25(OH)D concentration improvement, with no statistically significant change in PTH.45
In a more recent RCT, Thimachai et al. evaluated the effect of high dose ergocalciferol supplementation compared to K/DOQI recommendation. Sixty-eight patients were randomized into 2 groups, one under the conventional scheme, and another had the same regimen but twice the ergocalciferol dose. At 8 weeks of supplementation, the authors observed a significant improvement in 25(OH)D levels in both groups [from 20.99±6.68 to 33.41±8.92ng/mL (p=0.001) in the high dose group and from 20.84±7.21 to 23.42±7.89ng/mL (p=0.026) in the conventional regimen group]. In relation to plasma PTH concentration, it decreased significantly from 90.75±67.12 to 76.40±45.97pg/mL (p=0.024) in the high dose ergocalciferol group, and no significant changes were observed with the conventional group. In addition, the percentage of patients reaching plasma 25(OH)D concentration >30ng/mL was significantly higher in the high dose group than in the conventional dose group (60% vs. 19%, p<0.05). It should be noted that although a duplicate dose of ergocalciferol was used, no adverse effects were observed including hypercalcemia or hyperphosphataemia.36
Different dosages of cholecalciferol were also tested in prospective trials. Oksa et al. in a RCT, which involved 87 chronic kidney patients, stages 2–4, it was compared vitamin D supplementation with two doses of cholecalciferol, one low (5000IU/week) vs. one high (20,000IU/week), on mineral metabolism, vitamin D status and PTH plasma concentration. After 12 months, the authors found a significant increase in plasma 25(OH)D concentration in both groups [from 15 to 28ng/mL (p<0.001) in the low dose group and from 16 to 37ng/mL (p<0.001) in the high dose group]. The increase in the high dose group was statistically higher than that observed in the low dose group (P<0.01). Most patients receiving low dose cholecalciferol did not reach the reference value according to K/DOQI, while approximately 75% of the patients from high dose cholecalciferol group reached that value, without significant changes in calcium and phosphorus. Regarding plasma PTH concentrations, the authors found significantly decrease in both groups [from 63 to 48pg/mL (p<0.001) in the low dose group and from 50 to 40ng/mL (p<0.001) in the high dose group], without significant differences between the groups. So, a high dose of cholecalciferol was only more effective at increasing plasma 25(OH)D level.46
The studies with cholecalciferol supplementation reproduced better results in increasing plasma 25(OH)D concentration, and more often it reached the reference value of 30ng/mL after the supplementation scheme. Wetmore et al. conducted a RCT in which 44 pre-dialysis chronic kidney patients were compared with equal weekly doses of 1250μg (50,000IU) ergocalciferol or 1250μg (50,000IU) cholecalciferol over a period of 12 weeks, assessing its influence on concentrations of 25(OH)D, 1,25(OH)2D and PTH. Plasma 25(OH)D level was further evaluated 6 weeks after completion of therapy. The authors found that cholecalciferol was more effective at increasing plasma 25(OH)D concentration while supplementation was occurring [mean change in 25(OH)D concentration was 45.0±16.5ng/mL (p=0.01) in the cholecalciferol group and 30.7±15.3ng/mL in the ergocalciferol group]. At the end of supplementation, concentration of 25(OH)D decreased with both supplements, without differences between them. This suggests that these patients require continued supplementation to maintain serum 25(OH)D level. No significant differences were found in plasma concentration of 1,25(OH)2D in both groups. The authors verified that in the cholecalciferol group, there was an increase in 1,25(OH)2D3 portion and reduction of 1,25(OH)2D2 portion. The inverse occurred in the ergocalciferol group, explaining why there was balance in total plasma concentration of 1,25(OH)2D. Regarding the change obtained in PTH levels, patients in the cholecalciferol group had a greater reduction than patients treated with ergocalciferol (mean change PTH −15.3 vs 2.3pg/mL, p=0.02), however, a difference found in the baseline PTH values at the beginning of the study, makes this interpretation more complex from statistical point of view.47
Despite these studies promising results, a review published by Agarwal and Georgianos included 4 RCTs performed in pre-dialytic patients submitted to cholecalciferol supplementation and there was not any change in PTH level relative to placebo. Thus, the authors concluded that vitamin D supplementation in pre-dialysis CKD in order to reduce plasma concentration of PTH is not yet justified by current evidence.48
Calcifediol is a product already hydroxylated at C25, serving as the direct substrate for 1-α-hydroxylase. Calcifediol has been suggested as an effective therapy to increase 25(OH)D levels in general population, and its application in CKD is relatively recent. There are two formulations of this compound: a simple one, which causes a rapid increase in 25(OH)D concentration and a rapid catabolism by stimulated expression of FGF-23; and an extended-release (ER) formulation which leads to a more progressive and effective increase in circulating 25(OH)D levels.49
Sprague et al. conducted a double-blind RCT to evaluate the effect of ER calcifediol on SHPT treatment, which enrolled 78 patients with pre-dialysis CKD, mean GFR of 38.9±10.01mL/min/1.73m2, serum PTH levels >70pg/mL and 25(OH)D <30ng/mL. For 6 weeks, patients received daily treatments of ER calcifediol doses of 30, 60 and 90μg/day or placebo. The authors found that plasma 25(OH)D concentration increased in proportion to the administered dose, reaching after 6 weeks of therapy 37.3±2.0ng/mL and 84.8±5.5ng/mL on 30 and 90μg/day groups of ER calcifediol, respectively, and reduced by an average of 1.9±0.7ng/mL in combined placebo group. Differences between 3 supplementation groups and placebo were statistically significant (p<0.0001). Regarding the plasma PTH level, it decreased from the mean baseline value (140.3pg/mL) by about 20.9±6.2%, 32.8±5.7% and 39.3±4.3% in groups submitted to 30, 60 and 90μg/day of ER calcifediol, respectively, and increased 17.2±7.8% in combined placebo group (p<0.005). Despite a single episode of hypercalcemia, no hyperphosphatemic or hypercalciuric episodes were detected, therefore, authors concluded that oral supplementation with ER calcifediol is a safe and effective strategy for the treatment of SHPT associated with vitamin D insufficiency.19
Recently, to further examine safety and efficacy of ER calcifediol, Sprague et al. published a multicenter study composed by two 26-week randomized, double-blind, placebo-controlled trial and a subsequent 26-week extension, enrolling 429 subjects with stage 3 or 4 CKD, SHPT and vitamin D insufficiency. Subjects were randomized 2:1 to receive oral ER calcifediol (30 or 60μg) or placebo once daily. After treatment, more than 95% of patients receiving ER calcifediol achieved serum 25(OH)D concentration >30ng/mL. The ER calcifediol replacement also reduced plasma PTH by at least 10% in 72% of the patients, and reductions ≥ 30% increased progressively with treatment extension, achieving 50% at 52 weeks. The authors concluded that lowering PTH with ER calcifediol was independent of CKD stage and not associated to adverse events, making ER calcifediol a safe and effective treatment for these patients.50
Vitamin D supplementation and cardiovascular endpointsCardiovascular disease (CVD) is the most common cause of mortality in patients with CKD and has been associated with vitamin D deficiency. In a meta-analysis, Pilz et al. verified that it occurs an increase of 14% in risk of all-cause mortality in patients with CKD by each 10ng/mL of reduction in vitamin D level.51 In another meta-analysis, Duranton et al. suggested that vitamin D replacement reduces cardiovascular mortality risk in 27% when administered CKD patients.52
Endothelial dysfunction happens early in patients with CKD and is associated with origination of atherosclerosis and future cardiovascular events.53 Vascular calcification is a common complication in CKD and promotes an increase on cardiovascular morbidity and mortality rates.
Chitalia et al. investigated the relationship between vitamin D levels and endothelial function in nondiabetic with mild to moderate CKD patients. Endothelial function was evaluated by endothelium-dependent brachial artery flow mediated dilation (FMD), defined as the maximum percent increase in vessel diameter during reactive hyperaemia. The authors showed that patients with serum 25(OH)D level ≤15ng/mL had lower FMD when compared to patients with 25(OH)D level >15ng/mL (p=0.007). A direct association between vitamin D deficiency and low FMD was found (r=0.44; p=0.001). A multivariate regression analysis indicated an independent association between low vitamin D levels and low FMD, even after adjustments for traditional cardiovascular risk factors, such as age, gender, smoking, hypertension and hyperlipidaemia.53 Also, Capusa et al. in their recent cross-sectional study concluded, in 87 clinically stable CKD patients, that hypovitaminosis D is associated with subclinical peripheral arterial disease, independently of other traditional or non-traditional risk factors for atherosclerosis. A univariate analysis identified a correlation between serum concentration of 25(OH)D <15ng/mL and increased aortic calcifications scores (rs=−0.23; p=0.03); and through a multivariate binomial logistic regression models adjusted for cardiovascular risk factors, lower levels of 25(OH)D were considered an independent predictor for pathological ankle-brachial index (beta 0.84; 95% CI of beta 0.71–1.00; p=0.05).54
There are few studies about vitamin D supplementation and endothelial dysfunction. Recently, Kumar et al. published a randomized, double-blinded, placebo-controlled clinical trial in which 120 adult subjects with nondiabetic CKD stage 3–4 and serum 25(OH)D level ≤20ng/mL were randomized to receive either two directly observed oral doses of cholecalciferol (300,000IU) or matching placebo at baseline and after 8 weeks. Cholecalciferol treatment significantly improved vascular function as increased FMD in this group vs. sustained in the placebo group (between-group difference in mean change 5.49%; 95% CI 4.34% to 6.64%; p<0.001) and changes were correlated with 25(OH)D levels. In addition, endothelium-independent nitroglycerine mediated dilatation (NMD) and pulse wave velocity (PWV) also improved after intervention [between-group difference in mean change: 2.85% (95% CI 1.41% to 4.84%; p<0.001) and −1.24m/s (95% CI −2.16 to −0.74; p<0.001) for NMD and PWV, respectively].55
The NMD has been used as a control test for FMD. The PWV is a marker of arterial stiffness, atherosclerotic transformation and an independent predictor of CVD and mortality.55
Kumar et al. at the end of this study, tested the same intervention in placebo group and concluded that even in this group there were an improvement in endothelial function and vascular stiffness after cholecalciferol supplementation (mean change in FMD%: 5.8%; 95% CI 4.0% to 7.5%; p<0.001). NMD, PWV, PTH, FGF-23 and interleukin-6 also showed favorable changes.56
Levin et al. also assessed the vitamin D supplementation effect on PWV. In a randomized, placebo-controlled trial, the authors compared fixed doses of calcifediol (5000IU), calcitriol (0.5μg) or placebo thrice weekly on PWV, in 119 stable patients with CKD stage 3b-4. In spite of randomization, there were differences in baseline PWV values, so analysis took adjustments into account. After 6 months, PWV decreased in the calcifediol group (mean change: −1.1; 95% CI −2.2 to 0.1m/s), remained similar in the calcitriol group (mean change: 0.2; 95% CI −0.9 to 1.4m/s) and increased in the placebo group (mean change: 1.1; 95% CI −0.1 to 2.2m/s). The results of calcifediol versus placebo groups were statistically different (p<0.05). However, when baseline PWV was included as a covariate, there were not significant differences. Observationally, patients in the highest 25(OH)D tertile at the end of the trial had significant decreases in PVW (mean change: −1.0±1.0m/s) compared with the middle and lower tertiles (p<0.01). These results suggest that vitamin D supplementation decrease PWV in CKD patients, but more trials are needed to confirm these findings, because of attenuated effects when adjustments were done.57
Vitamin D may have a protective effect over arterial wall because it reduces smooth muscle cell proliferation and decreases vascular inflammation (by reduction of pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor and increases secretion of the anti-inflammatory cytokine, like interleukin-10). Thus, vitamin D seems to be crucial in the atherosclerotic process and an important target for treatment in the context of CKD, not only for bone and mineral regulation, but also for cardiovascular benefit.54
In contrast to these papers, Kendrick et al. promoted an RCT whose aim was to compare the effects of oral cholecalciferol and calcitriol on FMD. A total of 128 patients were enrolled with CKD 3b-4 and serum 25(OH)D levels <30ng/mL. They were randomized to receive either oral cholecalciferol (4000IU daily for 1 month, then 2000IU daily) or calcitriol (0.25μg daily for 1 month, then 0.5μg daily). The authors found no differences in FMD or NMD after 6 months of treatment and neither changes in total vascular endothelial cell expression of NFkB or in inflammation markers.58
In fact, these recent RCTs were of extreme importance, persisting uncertainty about the effectiveness of vitamin D replacement on cardiovascular endpoints in CKD patients.
Regarding vascular calcification, subjacent factors remain under investigation, but it seems to result from an imbalance between factors that promote and inhibit calcification, under some pathological circumstances, such as uremia.59 Hyperphosphatemia and excess of calcitriol accelerates an ostegenic transformation of vascular smooth muscle. Vitamin D has a paradoxal effect over vascular calcification. Its action on regulation of mineral metabolism may promote calcification, but other functions, such as cell cycle regulation and inflammatory response modulation may have a preventive effect.60 Because of this good effect, some authors defend that reasonable doses of vitamin D could provide a survival benefit for patients with CKD.55,60 Also KDIGO guidelines recommend the use of calcitriol or vitamin D analogs not routinely in pre-dialysis patients, but reserve them for treatment of severe and progressive SHPT to prevent episodes of hypercalcemia and vascular calcifications.7
More investigation about vitamin D supplementation is needed to understand the best supplement and recommended doses to avoid adverse events like vascular calcifications.
Concerning hard clinical outcomes, to our knowledge, only a retrospective study of Lishmanov et al. was conducted to analyze if vitamin D replacement can decrease the incidence of cardiovascular events. The study enrolled 126 men, average age of 70 years, CKD stages 3–4 with serum 25(OH)D level <30ng/mL. After 6 months of ergocalciferol supplementation, according to modified K/DOQI guidelines, the patients whose serum 25(OH)D level was increased by 25% from baseline were included in treatment group (n=90). Others patients who did not respond to treatment were considered as controls (n=36). At 27.2 months, the treatment group had fewer CVD events compared to the control group (21% vs. 44%, p=0.001). A multivariate logistic regression analysis estimated an odds ratio for 25(OH)D replacement status of 0.37 (95% CI: 0.14–1.0; p=0.05), after adjusting by age, baseline PTH, statin use, CVD history, diabetes and GFR. Both overall survival and CVD-specific survival were higher in the treatment group compared to control with Kaplan–Meier survival curves statistically different (Log rank P values of 0.008 and 0.02, respectively). Although there were some limitations, as a retrospective study, a relatively small group of patients, possibility of unmeasured confounders and the fact of treatment group having a lower history of diabetes compared to controls (53% vs. 73%, p=0.02), the authors concluded that vitamin D supplementation with ergocalciferol seems to be associated with significant reduction in cardiovascular events in patients with moderate CKD.61
Of importance, there are no controlled or observational prospective studies demonstrating that native vitamin D supplementation decreases mortality or hospitalizations. Other outcomes such as fractures, infections and neoplasias have also been proposed but remain to be confirmed.
ConclusionVitamin D insufficiency or deficiency are common in CKD, in spite of lack of reference levels of serum 25(OH)D. Vitamin D replacement has improved serum 25(OH)D and PTH levels, and it was thought to guarantee vitamin D pleiotropic functions, due to extra-renal hydroxylation. Despite these advantages, there was no consensus about the optimal threshold that must be achieved, and which supplement and dosage must be chosen. Uncertainty remains about effectiveness of vitamin D supplementation on endothelial dysfunction. Several guidelines exist on the topic, but also highlight the need of further investigation, because data is often poor and inconsistent.
Conflicts of interestThe authors declare no conflict of interest.