After contextualizing the generic frameworks of nanotechnology and nanomedicine, the 2 disciplines are discussed in the field of Nephrology. The potential downside to nanonephrology is the renal clearance of nanoparticles, the use of which is ever-increasing both for nanomedicinal purposes and in nanofoods. The positive impact of nanotechnology in Nephrology is centered on the development of renal nanodiagnostics for basic renal function studies, the early diagnosis of acute kidney injury, reliable and simple follow-up of chronic kidney disease and the improvement of magnetic resonance imaging. Renal drug nanotherapies comprise an important and dual-faceted area: the protection of drugs and nephrotoxic agents (e.g. antibiotics, antiretrovirals, contrast media, etc.) on the one hand, and the development of new kidney disease medications on the other. Renal ‘nanotheranostics’ is a promising but little-studied area. The impact of nanostructured supports on renal tissue regeneration is also discussed. The article concludes with a brief analysis of the various nanonephrology perspectives.
Después de una contextualización en los marcos genéricos de la nanotecnología y la nanomedicina, se exponen las 2 connotaciones nanotecnológicas de la Nefrología. La potencial faceta negativa de la nanonefrología es el aclaramiento renal de las nanopartículas usadas con fines nanomédicos o ingeridas en los nanoalimentos, cada vez más abundantes. El impacto positivo de la nanotecnología en la Nefrología se centra en el desarrollo de nanodiagnósticos renales para estudios básicos de la función renal, diagnóstico precoz del fallo renal agudo, seguimiento fiable y simple de la enfermedad renal crónica o la mejora de las imágenes de resonancia magnética nuclear. Las nanoterapias renales con fármacos es un tema de importancia que tiene 2 connotaciones: la protección de fármacos y agentes nefrotóxicos (ej. antibióticos, retrovirales, medios de contraste, etc.) y el desarrollo de nuevos medicamentos para enfermedades renales. La nanoteragnosis renal es una línea prometedora poco desarrollada. Se explicita también el impacto de los soportes nanoestructurados en la regeneración tisular renal. El artículo finaliza con un breve análisis de las perspectivas de la nanonefrología.
This new technology refers to the events and elements that take place in the so-called nanometric scale (from 1 to 100nm). However the breaking character of nanotechnology is not just a matter of size since some materials drastically change their properties when changing from micro to nano size (e.g., yellow gold is green, blue, red at nanometric size due to the effect called surface plasmon). Nanotechnology provides substantial added value in a wide variety of areas such as health, energy, home, mechanics, architecture, sensors, art, agriculture-foods, sports, toxicology, sex, among others. Nanotechnology is therefore a revolutionary technology with an impact similar to that of steam engine (XVIII and XIX centuries), electricity (XX century) and computing (XX-XXI centuries).1 In the 21st century hundreds of thousands of scientific articles have been published, thousands of patents have been registered, more than two thousand nanotechnology start-up companies have been created and 42 new journals have appeared with the prefix “nano” in their title. A current summary of nanoscience and nanotechnology may be found in the book by Kumar and Kumbhat2 published in 2016.
NanomedicineThis is the aspect of nanotechnology with the greatest economic impact and social repercussion.3 Prestigious organizations such as the WHO (UN) and the NIH (USA) consider it as a revolution in the Health Sciences.
The essential objective of nanomedicine is the development of nanotechnology tools for the early diagnosis, prevention and treatment of diseases as relevant as cancer, diabetes, cardiovascular and neurodegenerative disorders.
The multidisciplinary nature of nanomedicine is undeniable. It is not only the obvious combination of medicine and nanotechnology, it is essential the participation of other areas such as chemistry, biochemistry, molecular biology, genetics, as well as physics and engineering. In this context, it is curious to note how difficult is to clearly differentiate nanotechnology from biotechnology.
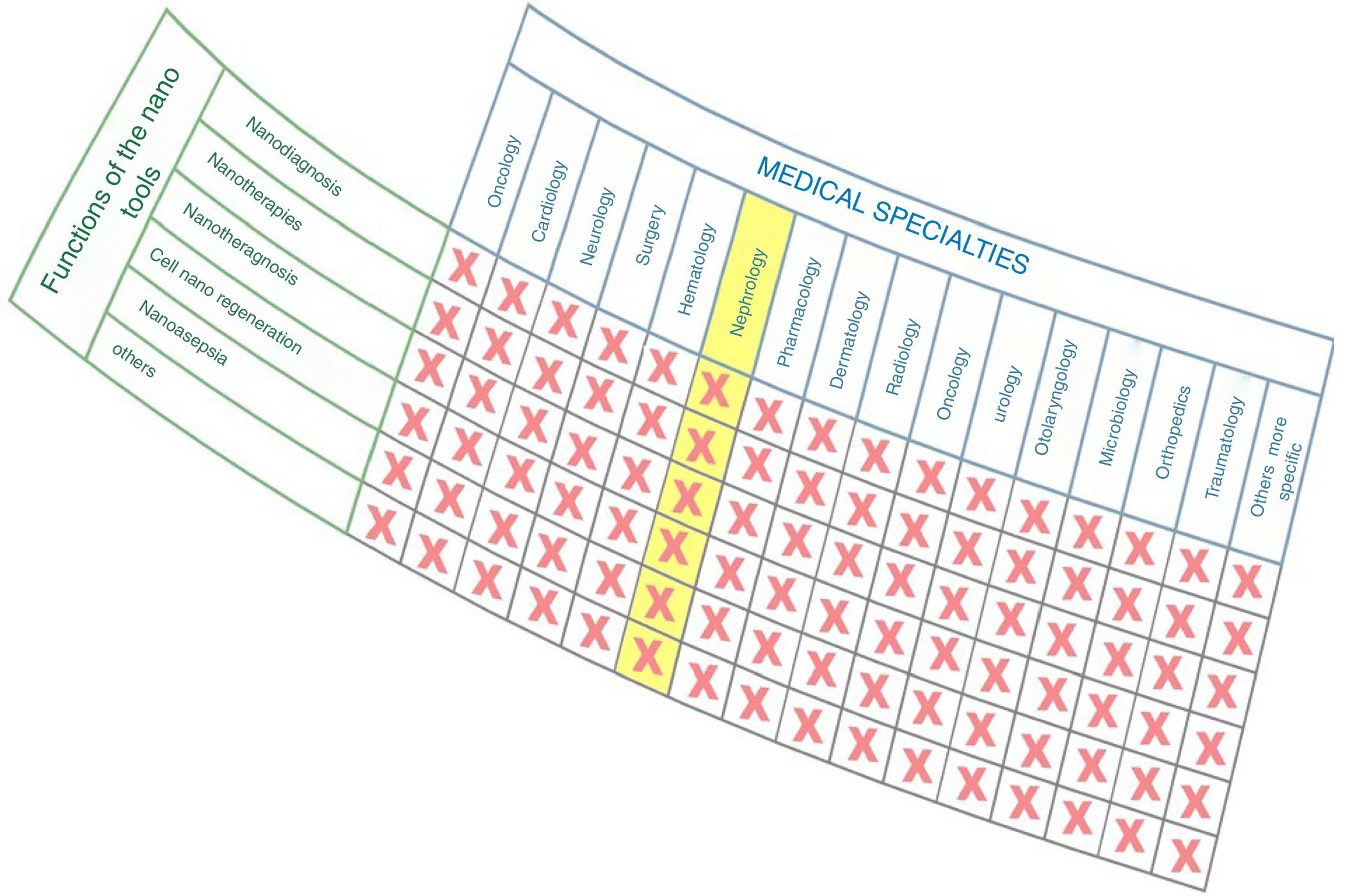
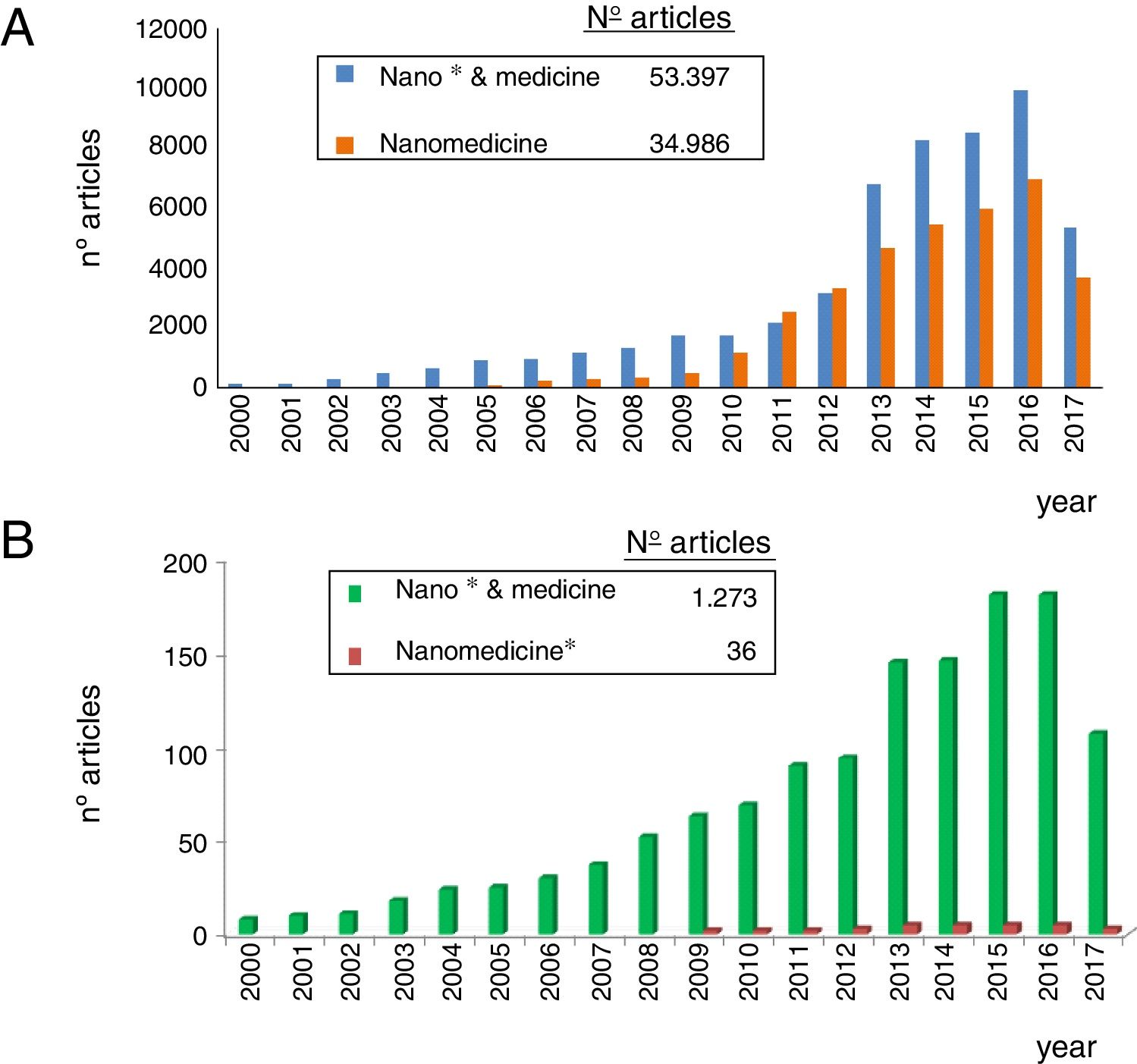
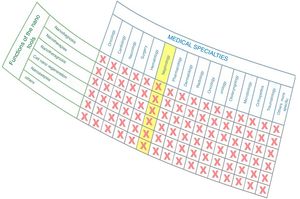
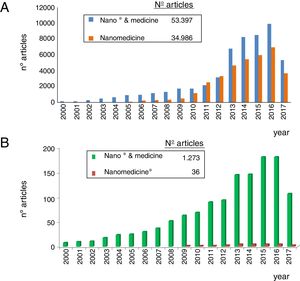
As observed in Fig. 1, nanomedicine can be organized by medical specialties (e.g. nano-oncology, nanodiabetes, etc.) or by the functions performed: nanodiagnosis, nanotherapies, nanoteragnosis, nanoasepsia etc. Obviously there is a correspondence between them. For the categorization and description of nanomedicine it is more useful the criterion of the functions of the nanotechnologies which are transversal to all medical specialties; the other option would involve the artificial description of closed compartments. For this reason, it is convenient to describe the impact in each medical specialty by showing the functions that can be developed, as it is done in this article in the field of Nanonephrology. The exponential and even linear growth of articles published in nanomedicine since 2013 is shown in Fig. 2A. There are more than fifty thousand articles published on the subject. Note that before the 21st century, there were practically no articles published in this subject. The difference between two searches is established in the international scientific database of the WOS of ISI: articles that have the prefixes “nano” and “medicine” (blue color) and those that have the term “nanomedicine” (red color). It is noted that more than 70% of the articles use this last term, which implies that it is already in the process of definitive consolidation in the scientific literature. In fact nanomedicine is being object of dissemination in a separate book of the collection “Frontiers of Science” sponsored by “National Geographic” recently seen in kiosks in Spain.
The representation of the number of articles published on nanonephrology during the 21st century is shown in Fig. 2B. From this graph it can be deduced that: (i) the number of articles of this specialty is only 2.5% of the total articles on nanomedicine; (ii) the growth of articles in nano-nephrology is exponential with a three-year biannual step; and (iii) the term nanonephrology is still far from being consolidated (it is only used in 3% of articles).
Renal clearance of nanoparticlesIt is a potential downside of nanonephrology. In the absence of renal clearance or biodegradation in inert compounds, nanoparticles used for diagnostic or therapeutic purposes can accumulate in the body and according to their nature they may produce toxicity (for example, many of the nanoparticles contain heavy metals or fluorescent agents). This fact limits the use of nanotechnology tools in the context of nanomedicine. The article by Choi et al.4 published in 2007 with more than 2400 citations is considered a milestone in the topic.
Thereafter, during the last 10 years, many articles have been published on specific nanoparticles. A recent article, in 2017, addresses the issue of the renal clearance of noble metal nanoparticles.5 The clearance of nanoparticles depends on its characteristics: nature, shape, crystallinity, hydrodynamic size, stability and, above all, surface activity (reactive groups and charges). Therefore, nanoencapsulation is a very promising strategy in nanomedicine since the coating (e.g. PEG, cysteine, dendrometers, liposomes, etc.) of a nephrotoxic drug (e.g. retrovirals, chemotherapeutic agents, anti-inflammatory agents, etc.) may prevent kidney damage. There is a fairly accepted general rule, with some exceptions: renal clearance of nanoparticles is acceptable if the hydrodynamic diameter is less than 5–15nm, which improves if they are biochemical (e.g. biopolymers) and organic (e.g., liposomes and dendrimers) nanoparticles. As an example, after intravenous injection of Quantum Dots labeled with Tc99m, those with a small hydrodynamic diameter (4.36nm) are cleared within a half-life of 48minutes, while those of greater hydrodynamic diameter (8.65nm) have a half-life of 20h.5 Have in mind that the hydrodynamic size of the inulin is 3nm.
The presence of nanoparticles in the human organism may be due to:
- •
Direct, voluntary and, therefore, controlled incorporation of nanoparticles as nanotechnology tools for specific purposes in the field of nanomedicine: therapeutic (e.g. carbon nanotubes), diagnostics (e.g. quantum dots for bioimages), teragnostics (e.g. gold nanoparticles), asepsia (e.g. silver nanoparticles).
- •
Unintentional and uncontrolled assimilation of nanoparticles due to a wide variety of reasons: water and air contamination with nanoparticles, ingestion of nano nutrients,physical contact with technology-based products (e.g. cosmetics), etc.
There are many mechanisms whereby nanoparticles are eliminated by the kidneys but the main mechanisms are two: direct, glomerular filtration of nanoparticles, and indirect, elimination of nanoparticles biodegradation products. A second route of elimination is through the liver which is designed to capture and eliminate nanoparticles of 10–20nm of hydrodynamic diameter (as an example, viruses), but this is a much slower process than the renal route.
Renal nanodiagnosticsThis is the first application described in this article on the use of nanotechnology tools that contributes to improve the field of nephrology. The application of nanodiagnostics in nephrology have clearly improved the ability to monitor with precision kidney diseases even in their early stages.
The use of nanotechnology to study the renal functionStudies on renal function, in vitro (artificial simulations of biological systems), ex vivo (experiments on tissues or cells extracted from a living organism) and in vivo (experiments on animals and humans) are much more efficient if they are based on nanotechnology. The results and the information obtained (placed in context and sufficiently discussed to help decision making), are the platform for advances in all fields of nephrology. Studies in vitro should be followed by in vivo experiments, and the generation of new drugs and systems for the treatment of patients; just following the classical sequence of R+D+T. One of the most relevant contributions in this context has been developed by the Wyss Institute of Harvard University under the direction of Professor Donald Ingber. Micro is combined with nanotechnology in a generic approach called “organs-on-chips” (OOC)6,7 applicable to the study of kidney diseases and other diseases in general. The OOC are platforms of polymers transparent with micro-channels in the bottom to introduce and evacuate fluids to a central platform that contains a strip of 4mm wide and 3–5cm long of a nano scaffold where cells are grown. This platform is being continuously observed by a fluorescence optical microscope, which allows to obtain real time high resolution images of cells when different agents (e.g. toxins, drugs, etc.) are passing through. This device allows to perform more efficient ex vivo studies aiming to discover new drugs, the prevention of kidney damage, evaluate nephrotoxicity of drugs, identification of biomarkers, evaluate molecular mechanisms, etc., without the need for animal experimentation. This platform won the National Award for Industrial Design in the USA in 2015.
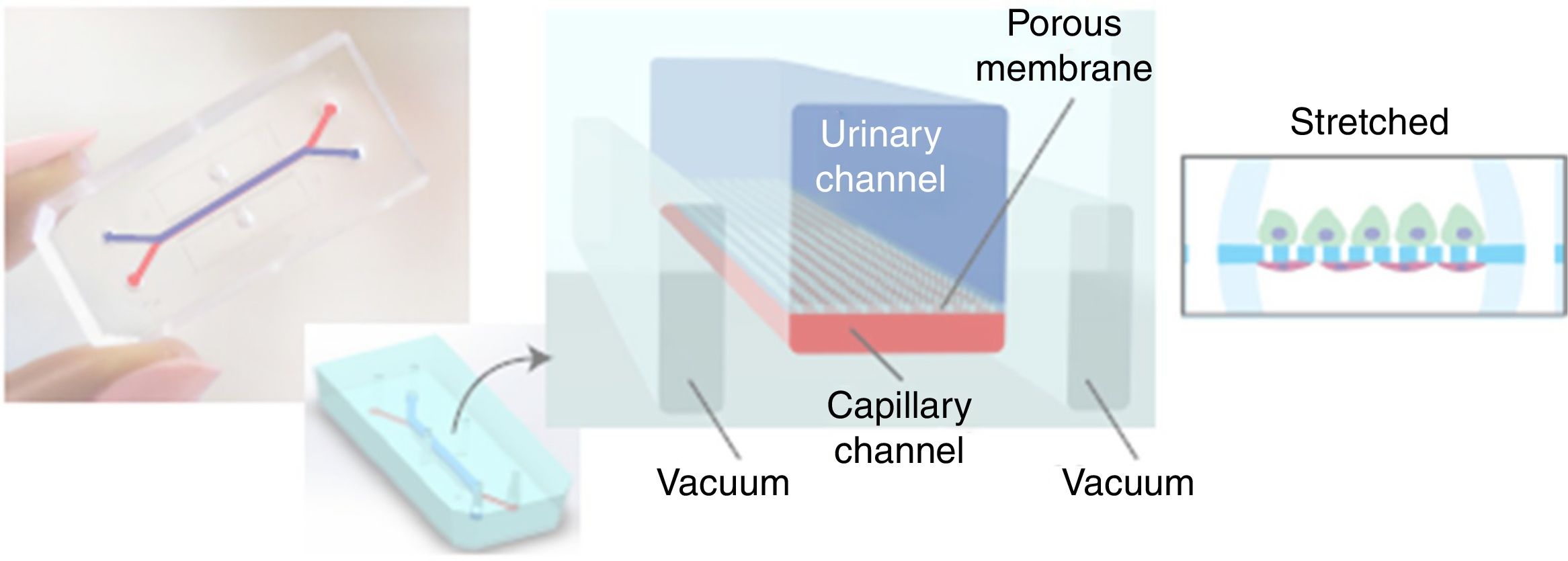
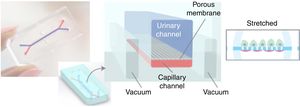
The same medical team, from Harward University, has just announced in June of 2017 the generation ex vivo of a renal glomerulus in a more specific approach than the previous one. Fig. 3 shows a diagram of this glomerulus on a chip.8 The functional changes are visualized with a fluorescence optical microscope. The device is a microchip (organ-on-a-chip) with fluid circulating through micro channels replicating the urinary (in blue) and capillary compartment (in red) of the glomerulus. The glomerular basement membrane is reproduced using a nanoporous membrane with the laminin protein that is part of the extracellular matrix. The polydimethylsiloxane membrane is not rigid and must be stretched by a vacuum system that works cyclically. The “human” glomerulus incorporates cells derived from the primary culture of human endothelial cells and pluripotent stem cells are induced for transformation into podocytes. The real-time images obtained are spectacular and show the capacity of inulin and albumin to go through the membrane and the possibility of generating an in vitro model of podocytopathy by adriamycin nephrotoxicity.
Photographic and schematic representation of a microfluidic “organ-on-a-chip” with the urinary (blue color) and capillary compartments (red color) of the renal glomerulus. A flexible nanoporous silicone membrane separates both compartments. The cyclic vacuum assembly is necessary to make the artificial glomerular membrane rigid.
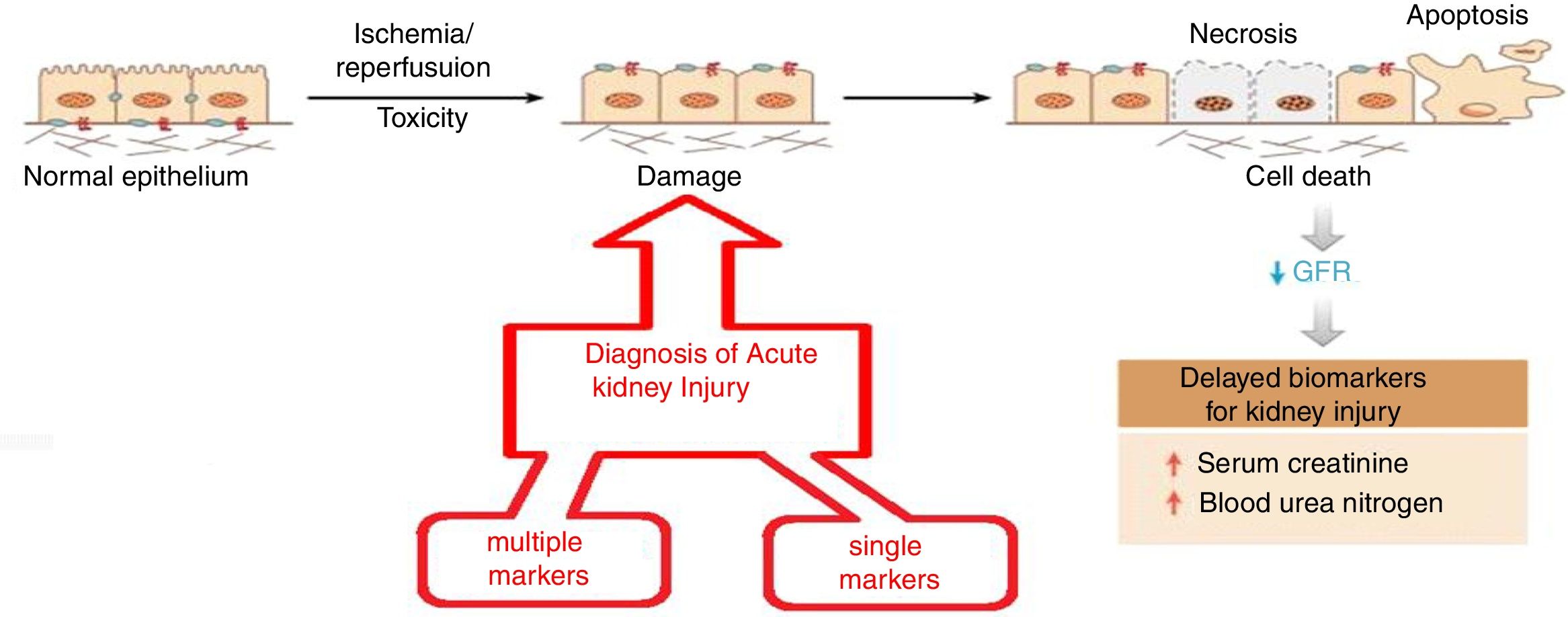
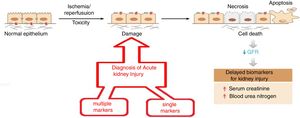
The objective is to apply nanotechnology tools to improve the diagnosis of common renal diseases. Some representative examples are presented. Early diagnosis of acute kidney injury is a challenge; as seen in Fig. 4 the normal epithelium is damaged by toxic agents or by ischemia/reperfusion. It is necessary to have an efficient indicator showing the beginning of these damages so appropriate therapy can be applied urgently. Otherwise, necrosis, apoptosis and cell death will occur. The rapid fall in renal function is evidenced by the marked increase in serum creatinine and blood urea, which certify tissue damage.
Evolution of acute renal failure. The time of application of two types of nanomarkers (simple and multiple) for early diagnosis.
As shown in Fig. 4, there are two diagnostic approaches. The multiple marker strategy9 that consists in measuring the relative ratio of 14 biomarkers in urine using a fluorimetric system based on a combination of microfluidics and nanotechnology with a chemometric treatment of the data; the diagnosis is binary and has a 99% reliability (there are no false negatives). A second strategy, a single marker10; it is based on a nano-reactive (immunosensor based on a nanoantibody obtained from the camel). This nano-reactive detects the lipocalin associated with a neutrophil-gelatinase which is an early marker of acute renal failure. The nano-reactive is immobilized on a titanium electrode and its detection is based on the electrochemical spectrum; the procedure is simple, fast and selective (compared to other macromolecules in urine) and has shown great precision and accuracy.
The detection and monitoring of chronic kidney disease (CKD) is routinely done by the measurement of blood markers. Nanotechnology offers ingenious solutions to address this type of diagnosis. Such is the case of the simple identification of the different states of CKD (from healthy individuals to those with advanced chronic renal failure) through direct breath analysis.11 This diagnosis is based on the fact that renal failure changes the proportion of more than 40 volatile organic compounds (VOCs). This is accomplished by the use of carbon coated nanotubes as sensors in the instrument called “electronic nose”, which is commercially available.
By using chemometric methodology of main components analysis (PCA) the results in more than 1000 individuals demonstrates a capacity for discrimination between CKD stages of 95% and that the differences in breath humidity do not significantly influence the results.
The morphology of the glomerulus can be quantified by nuclear magnetic resonance imaging (MRI-D) using gadolinium as a marker. Nanotechnology provides a nanomarker called nanoferritin that binds primarily to the glomerular membrane12 when given intravenously. It has a high renal selectivity and the toxicity caused by gadolinium is reduced. The results obtained (number and size of nephrons, renal volume, filtration rate, etc.) that help to define the factors that contribute to CKD are highly reliable. Currently, one of the most important nanomaterials for its applicability in medicine is graphene. Its applicability as a biosensor, by joining networks of gold nanoparticles, has made possible to create a portable sensor for glucose, temperature, humidity and pH in the sweat of diabetic patients. This system is also capable of subcutaneously administering drugs such as metformin through thermally activated microneedles of polymeric graphene.13
A biosensor based on a compound of MoS2-graphene (MG) allows the measurement of the parathyroid hormone concentration in serum from patients with kidney disease, linearly in the range of 10–1000ng/ml and has been validated against a commercial immunoassay of clinical use.14 Graphene may have a direct cytotoxic effect inducing cell necrosis and apoptosis,15 but its use as an oxide of graphene in the form of two-dimensional leaves is inert to the kidney and in animal models it has been shown that is filterable through the glomerulus without inducing injury to the podocyte, endothelial cell or any component of glomerular filtration barrier.16
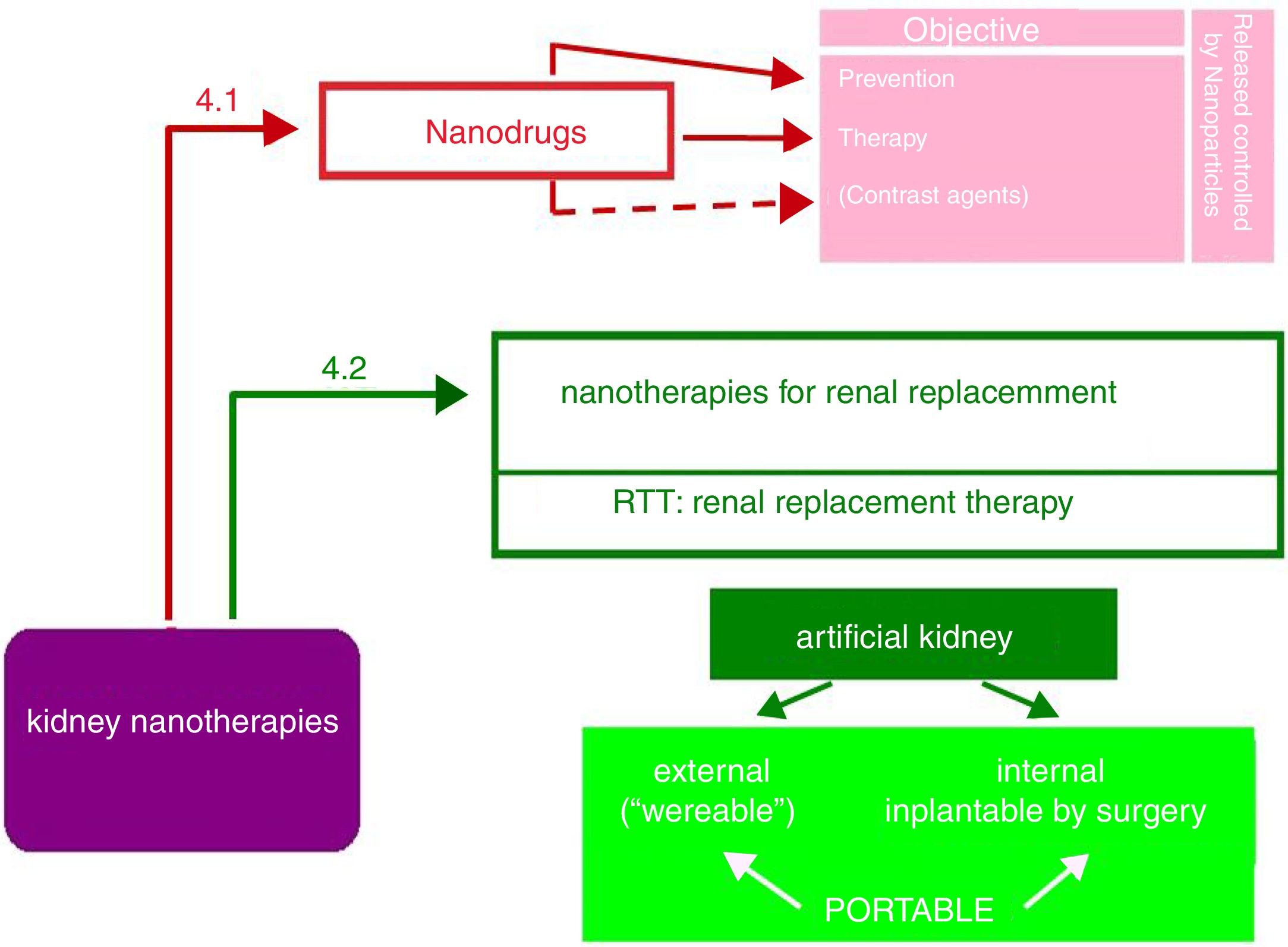
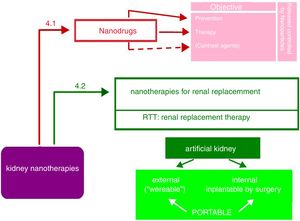
Nanopharmaceuticals in kidney diseaseAs shown in Fig. 5, the objectives of nanodrugs are the prevention and treatment of kidney diseases. Some authors17 also include the use of nano-compounds as image contrast media, although the authors consider these have diagnostic more than therapeutic use (e.g., nanoferritin in renal NMR12). Another application is the delivery of the drug in encapsulated nanoparticles able to control its release (liposomes, dendrimers, polyethylene glycol, etc.). This promising field of nanonephrology has not received enough attention from researchers and is a challenge that needs urgent attention.
Here we describe trepresentative examples of nanodrugs for renal protection and for the treatment of kidney diseases. The protection of the kidney by nanoencapsulation of nephrotoxic drugs such as contrast agents, antibiotics, retrovirals and anti-inflammatory drugs of frequent use but constrained to kidney patients is one of the most promising topics in nanomedicine. A characteristic example, developed by Serrano et al.,18 is the nanoencapsulation of amphotericin B with a polymer derived from cytosan, which enables the oral administration of antifungal, with a bioavailability of 24% and an efficacy similar to that of liposomal amphotericin administered parenterally, but reducing the renal exposure of the drug and therefore, its nephrotoxicity. This novel nanoparticle prevents the degradation of amphotericin B by the acidity of the stomach. The efficacy of this nanoencapsulation in the treatment of leishmaniasis, aspergillosis and candidiasis in animal models has been demonstrated. Renal damage (e.g., inflammation, fibrosis, cell death, etc.) could be efficiently treated with nanomedicines19,20; however, up to now this has not been satisfactorily demonstrated.
A more extensive and profound investigation is necessary to ensure that these nanotechnological-based drugs fulfill two objectives: controlled release from the nanoparticle and having the kidney as the target-organ (GTT: Glomerular Tubular Targeting). The use of hydrophilic polymeric nanoparticles that facilitate selective transport to the interior of the kidney is promising. A recent and excellent review on this topic demonstrates the importance of nanomedicine in the treatment of tumors and kidney diseases.17
Nanotechnologies for renal replacement therapyThe largest impact of nanotechnology in Nephrology is the development of a surgical implantable artificial kidney that would minimizes the need for kidney transplantation of cadaveric or living donors and reduce the demand for standard dialysis. This represents a milestone in the development of hemodialysis. The macrodialyzers of the considered “father” of hemodialysis have been incorporating scientific and technical advances such as miniaturization, automation and computerization; at the beginning of the 21st century hemodialyzers are undoubtedly smaller and much more efficient, more biocompatible and more self-controllable. Its design has even made possible to be used as home dialysis. The description of portable artificial kidneys in the literature is very diffuse and unclear. There are many short informative articles, without technical details and videos more focused in marketing than in scientific dissemination. The cause of this situation is that there are patents that prevent a clear description of new devices. Many aspects remain obscure, e.g. the duration of an artificial kidney implanted by surgery.
As observed in Fig. 5, the portable artificial kidney may be external or internal with respect to the organism to which it is incorporated.
The external artificial kidney is called “wereable”, it also means that it is “removable”. They are belts that incorporate all the elements of hemodialysis. Of course, they require a vascular access. Many authors agree that this external artificial kidney is not likely to succeed, although one of its latest models has been approved by the FDA. In 12–14h it performs the function of an ordinary dialyzer in 3–4h.21
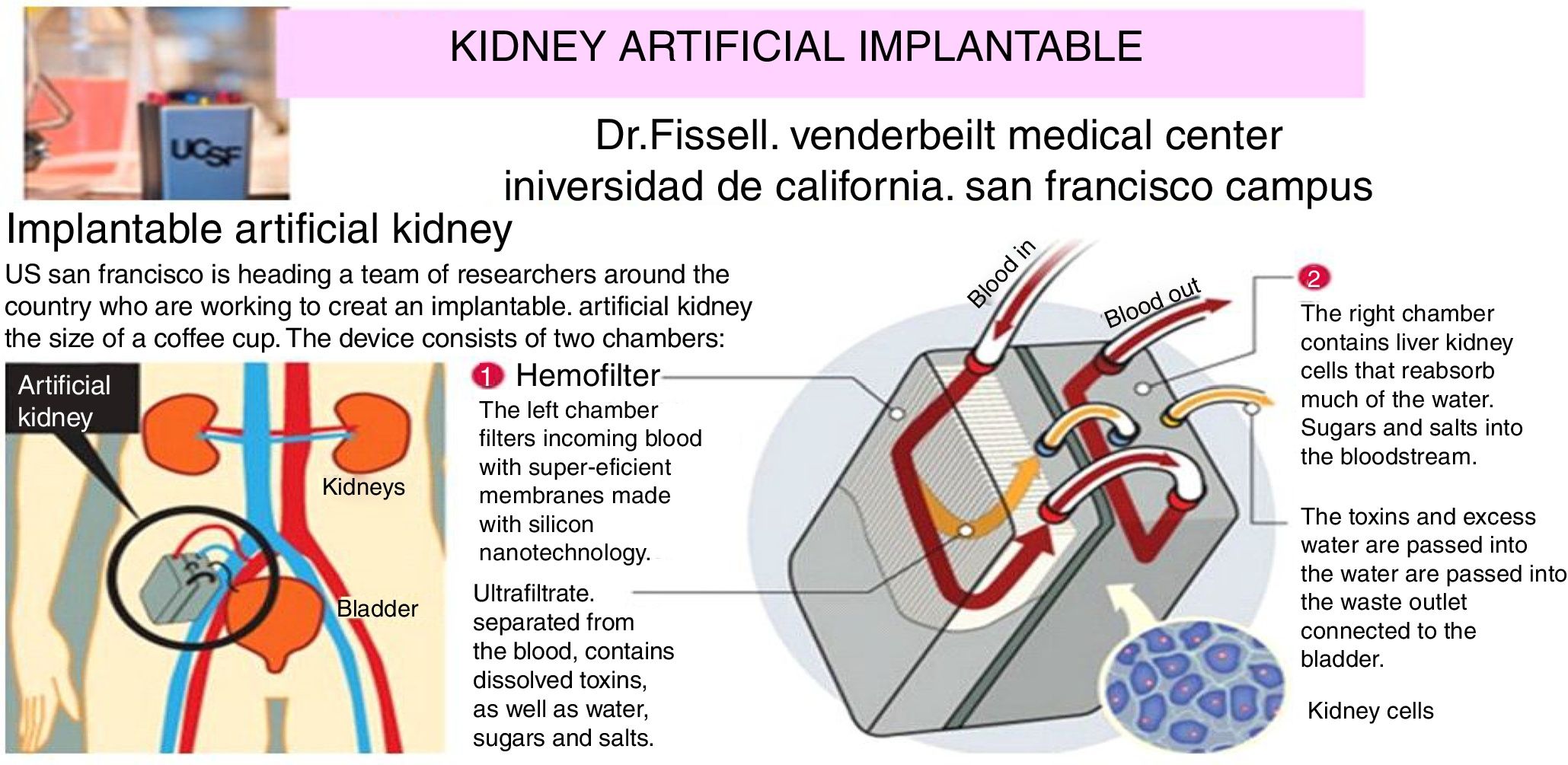
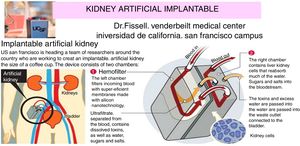
The internal artificial kidney is the one implanted by surgery, as if it was a “donated natural kidney”. Fig. 6 shows schematically the artificial kidney that is already in humans clinical trials (2017–2018) in the Venderbeit Medical Center at the University of California (San Francisco Campus) (UCSF)22,23 under the direction of Dr. Fissell. The artificial kidney has two compartments: (1) Nanoporous silica filter compartment that has: an input of the blood stream (in red), and two outputs: filtered blood and the ultrafiltrate (in orange) containing toxins, salts, water, sugars, etc. The silica nanotechnology membrane filters very efficiently substances that pass to the ultrafiltrate; and (2) Bioreactor compartment with living kidney cells that has: two inputs (the filtered blood stream and the ultrafiltrate coming from the previous compartment) and two outputs (the one from the treated blood to the renal cells that have reabsorbed the sugars, salts and part of the water, “the urine” connected to the bladder that contains toxins and excess water).
Artificial rhinoplasty with surgery under development at the University of California.
The same team from the University of California-San Francisco is developing the implantable kidney-in-a-chip.24 This is another artificial kidney that combines micro and nanotechnology using the same approach as the previous macro model: combination of filter nanotechnology and living kidney cells (VO Inside). To replicate a functioning kidney it is necessary to include 15 nanotechnological microchips that are stacked in a plastic compartment five times smaller than in the previous described artificial kidney. This technology is in its earliest stages, still far from animal and human trials. Miniaturization is very promising since it will allow surgical implant and the portability of an artificial kidney in a simple and comfortable manner.
Renal nanoteragnosisThe simultaneous combination, in situ and in vivo, of both, therapies and diagnoses is called teragnosis. It was developed at the end of the 20th century. The irruption of nanotechnology has given rise to nanoteragnosis,25–27 which is one of the most promising areas of nanomedicine. Nanoparticles of different types, inorganic (e.g. gold, mesoporous silica, etc.), organic (e.g., dendrimers, liposomes, carbon nanotubes, etc.) or biological nanoparticles (e.g. biopolymers) serve as nanocarriers able to transport the components inside or on its surface. The compounds that can be transported are therapeutic, diagnostic, orienting and antiplatelet agents; and, agents able to exclude other (macro) molecules in the bloodstream.
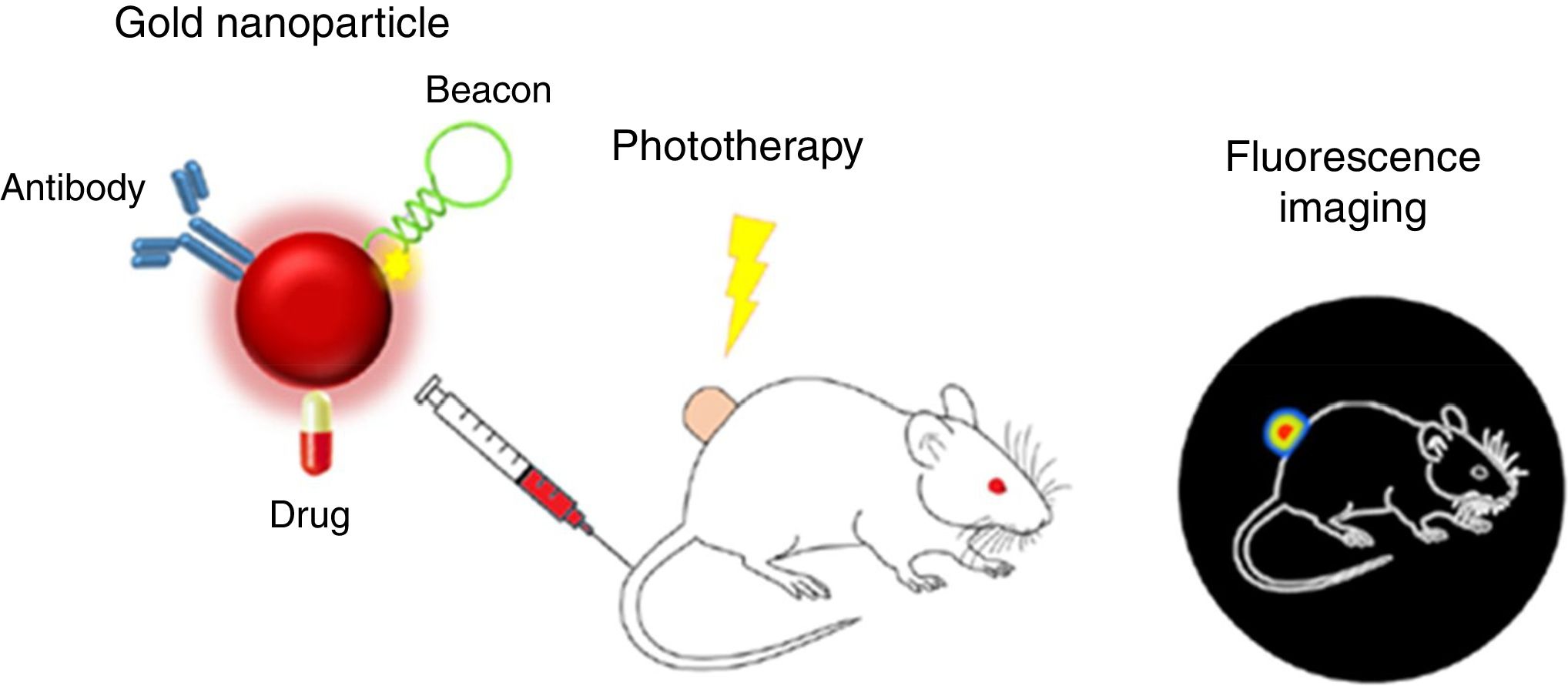
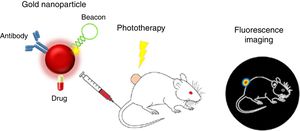
Proposals of nanoteragnosis in kidney diseases are scarce, which implies that there are opportunities for R+D+T for as long as specific nanodrugs for kidney disease are being developed. A spectacular example of nanoteragnosis for renal cancer28 is based on the use of gold nanoparticles (of red color due to 15nm size) as nanocarriers, which also convert the light energy of a laser into thermal energy and inhibit fluorescence of fluorophores on its surface. The directional target is an antibody that binds to renal tumor antigens and is “attached” to the teragnostic nanoparticle. The DNA hairpins that have fluorophores attached act as nano “lights”. As they reach the renal tumor they separate and an intense fluorescence is displayed. The therapeutic agent is a typical chemotherapeutic drug (such as 5-fluoracil). Fig. 7 shows how the nanoteragnostic agent is injected into the animal intravenously, anchors to the kidney tumor and fluorescence is generated by the detachment of the DNA forks. The chemotherapeutic effect of the drug is potentiated by an external laser beam directed to the fluorescent zone and the tumor temperature increases up to 45°C. It is a spectacular example of the combination of three therapies: the gene, the thermal and the chemistry.
Nanoteragnosis for renal cancer in a mouse.
One of the first cross sectional areas of nanomedicine (see Fig. 1) is tissue and cellular nanoregeneration through the use of nanotechnological structure supports (scaffolds) of different types: organic (e.g. carbon nanotubes, nanocellulose and derivatives), inorganic (e.g. quantum dots, mesoporous silica) or biological (e.g. use of clean kidney tissue as such.29
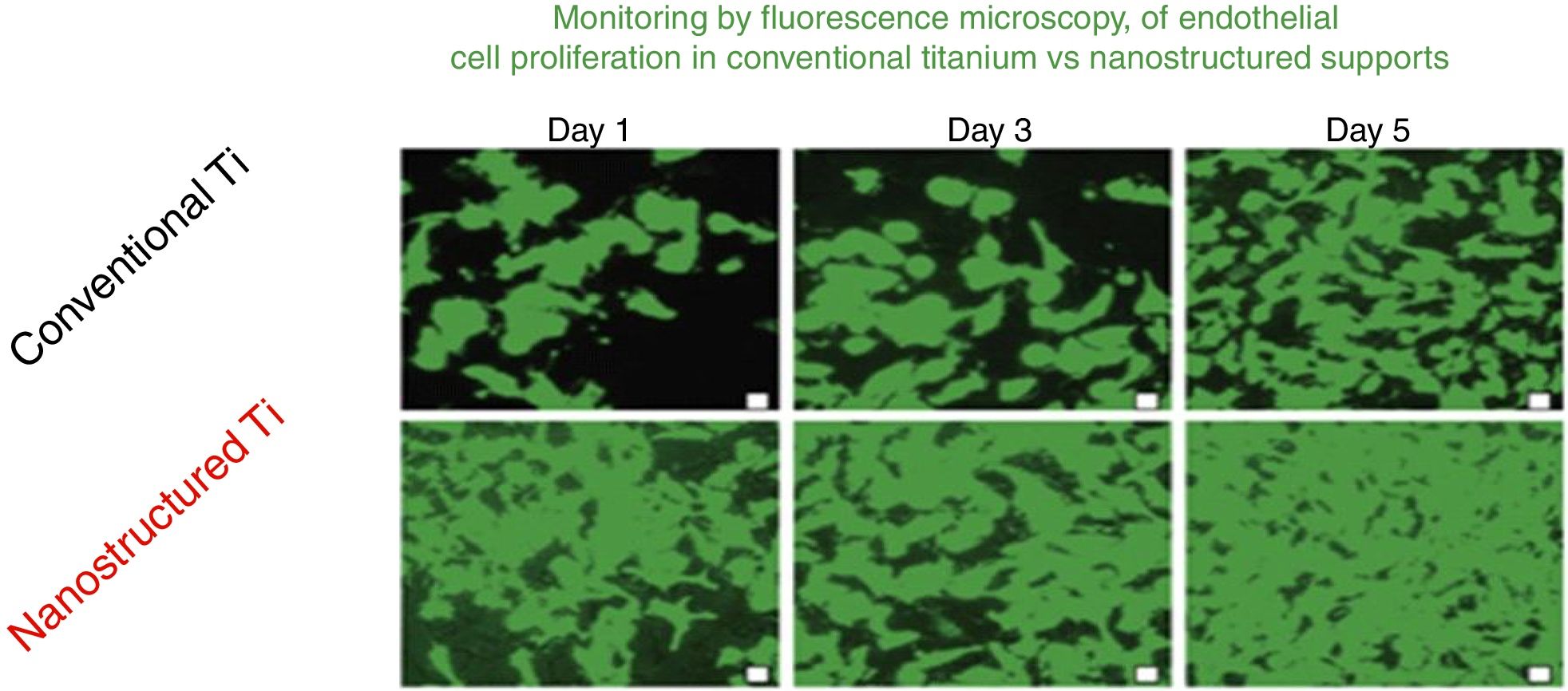
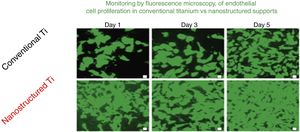
The advantages of nanotechnological scaffolds30 are: (A) high cell compatibility; (B) reproduces the extracellular native matrix; (C) promotes cell adhesion and proliferation; and (D) improves the properties (mechanical, electrical, magnetic, catalytic, optical) of the conventional micro structured supports. These new products are being commercialized already. An interesting review on nanotechnology applied to tissue engineering illustrates specific examples.31 An example related to the renal field is the growth of endothelial cells of the glomeruli capillaries.32Fig. 8 shows the monitoring by fluorescence microscopy, of endothelial cell proliferation during 5 days in conventional vs nanostructured supports. It is observed that the growth is greater in nanostructured than conventional support. In this context it is worth mentioning that the interest in stem cells has been invigorated thanks to the so-called stem cell nanotechnology (SCN)33 that has important implications: (1) isolation of stem cells by magnetic nanoparticles; (2) nanotubes of modified carbon to introduce genes into the stem cells; (3) supports of carbon nanotubes for stem cell proliferation and differentiation; and (4) nanoparticles (e.g., quatum dots) for reliable stem cells image analysis.
Comparison of the growth of endothelial cells in conventional supports and Nanostructured.
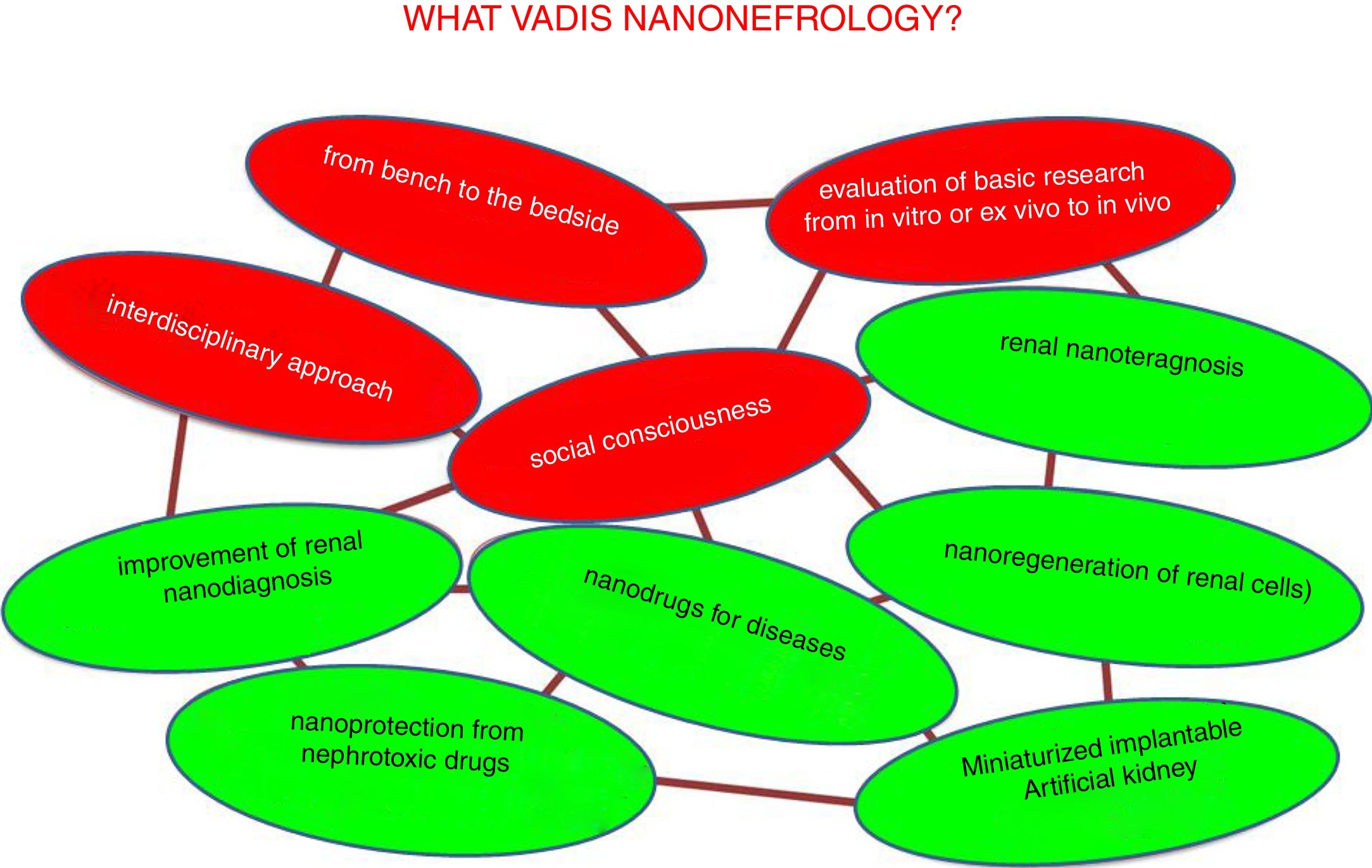
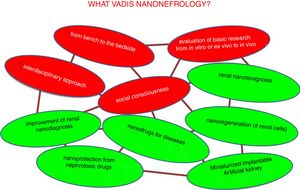
The desired future trends in nano-nephrology are shown schematically in Fig. 9. In fact, the fields to be developed are interrelated so they form a network. Colors distinguish between generic and specific areas of development. The four generic trends of nano-nephrology coincide to a large extent with those of nanomedicine (Fig. 9 in red). These are:
- 1.
Provide continuity to the I+D+T sequence so that the development of basic research is translated to the diseased patient. In this context, we recommend the lecture of Ionannides article of 2016 with the title “Why most clinical research is not useful?”34 that analyzes the causes of the deviation from the objectives that clinical research should have. This also occurs in other disciplines, such as chemistry.35 In the evaluation of research work, to much weight is placed in the amount of articles published (with extraordinary economic benefit of scientific publishers).36 This foster an environment in science and technology in which the number of publication predominates over its utility; this should be eradicated.
- 2.
As a consequence of the above, the translational aspects of nanonephrology studies should be reinforced. Going from in vitro or ex vivo research to in vivo, which should add value to the publication.
- 3.
The interdisciplinary approach of research (the scientific–technical interfaces) facilitates de application in patients of the great advances in nanonephrology. This characteristic is innate to nanomedicine
- 4.
Like nanomedicine, nano-nephrology offers very important contributions with huge economical impact. Therefore, it is essential to address the issue of social responsibility in coherence with generic trends in science and technology.37 It involves systematical consideration of issues as transparency, ethical behavior, accountability and compliance with current laws. All of this aimed at satisfying the needs of patients as a priority; they are the most relevant stakeholders, above the interests of doctors, hospitals, companies and governments.
The specific trends in nano-nephrology should be based on the materialization of the generic areas shown in Fig. 9. Most relevant are:
- •
Implantable long-term miniaturized artificial kidney.
- •
Clear improvement of diagnosis through nanotechnology.
- •
Renal nanoprotection of nephrotoxic drugs such as some antibiotics, retrovirals, contrast media for (TAC, NMR).
- •
Nanotech-based medications for use in specific disorders such as cancer, fibrosis, infections, etc.
- •
Development of renal nanoteragnosis.
- •
Efficient nanoregeneration of renal cells.
- •
Others, which will arise in the immediate future.
The purpose of this manuscript is not an intense review on nanonephrology with more than 1300 articles published at the end of 2017. The authors have selected the most representative material to offer an overview showing a generic “state-of-the-art and prospects” with the inclusion of illustrative examples.
Nanonephrology is still at the first stages of exploitation of the nano-tools able to improve diagnoses, therapies, and renal teragnosis, as well as promoting the study of renal function.
This article does not intend to convince readers that nano-nephrology is the panacea in Nephrology. The real intention of the authors is to open a window to a technology that can revolutionize current nephrology.
The authors want to target on young nephrologists so they became involved in a world of innovation with some guarantee to succeed in publications and improving Nephrology.
The enormous potential of the medical application of nanotechnology in the diagnosis and therapy is completed with the parallel development of the “omics” – proteomics, peptidomics, transcriptomics, genomics and metabolomics – whose application generates a huge amount of information that is impossible process with the ordinary means of data management. This is how big data was born and the need to exploit that exorbitant mountain of data that is generated daily in medicine, both in research and in digital patients records.38 It is estimated that the biomedical information available will double every 18–24 months during the next few years. The need to properly store and manage this data has caused the birth of two new concepts: data mining and machine learning. Data mining is a field of statistics and computing science, which tries to discover patterns in large volumes of data39 this is done through artificial intelligence and machine learning. Machine Learning is a branch of Artificial Intelligence where computers develop machine learning algorithms from databases – including millions of data – not being previously programmed. The linear regression or logistic regression is overcome by neurons networks, vectors (support vectors machines) and decision trees (random tree, random forest). These new data analysis systems are already being applied in the field of Nephrology.40 One of the challenges of exploitation of big data is to maintain the confidentiality of personal data in a secure environment, but at the same time accessible to integrate the information and be able to exploit them efficiently and productively. In conclusion, nanotechnology is a new technology that will contribute to the development of the “new” Nephrology. The future will go through the integration of nanodiagnostics, nanotherapies, “omics” and the adequate exploitation of huge amount of data that will be generated with these techniques.
Conflict of interestsThe authors declare that they have no conflicts of interest in relation to the text.
Miguel Valcárcel wishes to show his gratitude to the Andalusian Society of Nephrology (SAN) and the Spanish Society of Nephrology (SEN) for their support and confidence deposited on this author (chemist and nanotechnologist), to deliver lectures on the subject of this article during the year 2017 in Córdoba (April) and Burgos (October) respectively.
Please cite this article as: Soriano ML, Rodríguez-Benot A, Valcárcel M. Bases nanotecnológicas de una «nueva» Nefrología. Nefrologia. 2018;38:362–372.