Fabry disease is an inherited, X-linked lysosomal storage disorder caused by deficiency of the enzyme alpha galactosidase A (alpha-GLA A), which leads to glycosphingolipid accumulation, mainly globotriaosylceramide, in tissues. Disease prevalence and the index of suspicion are both low, which tends to result in delayed diagnosis and treatment.
We present the case of a male Fabry disease patient who manifested no angiokeratoma lesions but presented multiple parapelvic cysts and renal failure. The genetic study revealed an alpha-GLA A gene mutation that had not been recorded in the mutations registry. The de novo mutation was not found in his relatives and it was not transmitted to his offspring. The large number and peculiar appearance of the parapelvic cysts led to the diagnosis.
La enfermedad de Fabry es una enfermedad de depósito lisosomal de carácter hereditario, ligada al cromosoma X, causado por el déficit de la enzima alfa-galactosidasa A (alfa-GLA A), lo que conduce a la acumulación de glicoesfingolípidos, principalmente globotriaosilceramida, en los tejidos. Es una enfermedad poco prevalente, y con muy bajo índice de sospecha, por lo que, generalmente, existe un retraso en el diagnóstico y en el tratamiento.
Presentamos un caso de un paciente varón afecto de la enfermedad de Fabry, que presentaba múltiples quistes parapiélicos e insuficiencia renal, sin presentar angioqueratomas. El estudio genético mostró una mutación en el gen de alfa-GLA A que no ha sido descrita previamente en el registro de mutaciones, y de novo, ya que no se encontró en otros familiares, además que no fue trasmitida a la descendencia. La presencia de múltiples quistes parapiélicos y su peculiar aspecto fue lo que hizo sospechar el diagnóstico de la enfermedad.
Fabry disease (FD) is a result of a deficiency in the enzyme alpha-galactosidase A (alpha-GLA A), which leads to the deposition of globotriaosylceramide (Gb3) in the tissues.1 It is a hereditary disease, linked to the X chromosome, that manifests in homozygous men and heterozygous women, the latter being affected to a variable degree.2 It is a disease that may occur in all races, whose prevalence ranges from 1:50,000 in men3; however, a study conducted in newborns found an incidence of 1:37,000.4 FD has a broad range of clinical manifestations, which makes it difficult to establish a timely diagnosis. The onset of symptoms, in general, starts in childhood, and neuropathic pain and multiple angiokeratomas on the skin are present in a high percentage of cases.5 In adulthood, renal impairment begins, characteristically, by proteinuria with progression to chronic kidney disease, which is associated with an increase in mortality, in these patients. Parapelvic cysts have also been reported in FD as a radiological finding.6
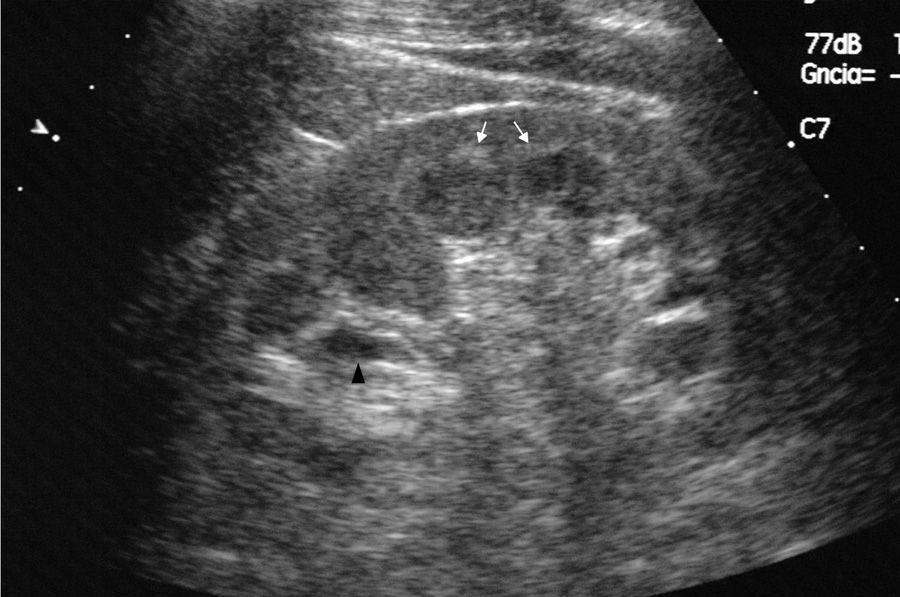
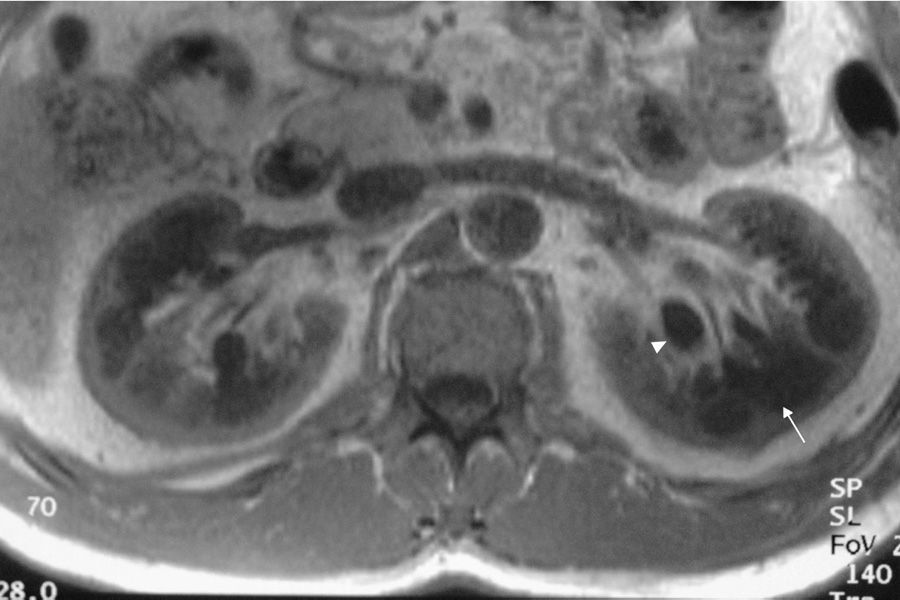
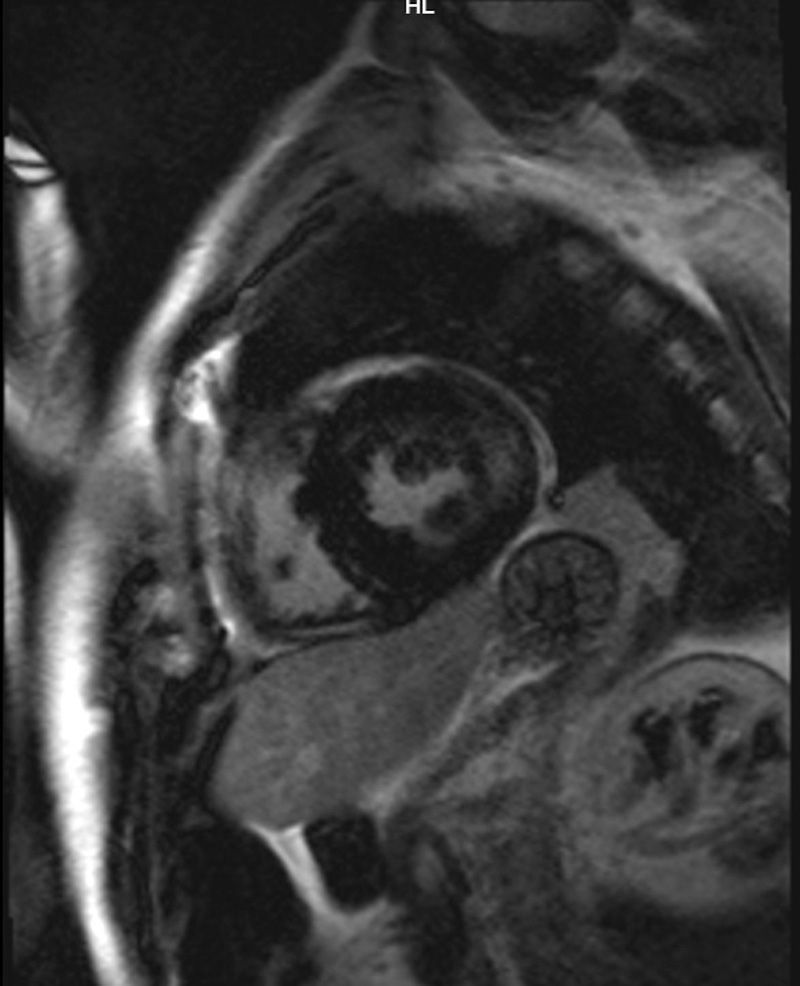
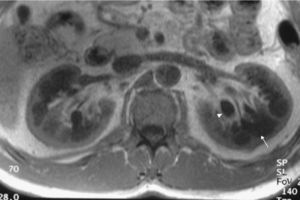
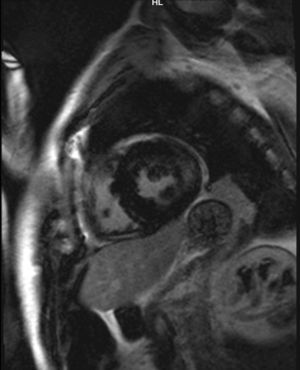
Case reportWe present the case of a 47-year-old man with no family history of FD who had been undergoing outpatient monitoring in our department since 2002 owing to proteinuria. In 2006, abdominal ultrasound and magnetic resonance imaging (MRI) showed poor corticomedullary differentiation and multiple bilateral parapelvic cysts (Figs. 1 and 2). In 2007, owing to a deterioration in kidney function with serum creatinine levels of 1.6mg/dl and proteinuria levels of 1g/24h, it was decided to perform an ultrasound-guided renal biopsy, which revealed changes consistent with moderate/severe chronic interstitial nephritis. In 2009, the patient was admitted owing to oedema and dyspnoea on exertion. The examination showed no cutaneous lesions suggestive of skin angiokeratomas. Laboratory testing on admission showed serum creatinine levels of 1.7mg/dl and proteinuria levels of 2.4g/24h. Echocardiogram and cardiac MRI findings showed probable myocardiopathy due to deposition disease (Fig. 3). Electromyography showed signs of small fibre neuropathy, with abnormalities in autonomic function. A sweat test was performed that collected no sweat after stimulation with pilocarpine, and an ophthalmological study was performed that revealed cornea verticillata.
Given the clinical suspicion of FD, the level of alpha-galactosidase activity in leukocytes and plasma was determined, using a 4-methylumbelliferyl α-d-galactosidase fluorogenic substrate. GLA activity was 0.5nmol/h/ml (2% of normal) in plasma, and 4.2nmol/h/mg for protein (10.7% of normal) in leukocytes, which confirmed a diagnosis of FD. The enzyme's activity was normal in the family members of the patient who were studied (mother and sister). A genetic study was performed wherein genomic deoxyribonucleic acid (DNA) was extracted from a dried blood spot and coding gene fragments were amplified using PCR. Direct sequencing of the 7 exons of the GLA gene was performed using a capillary sequencer (ABI PRISM® 310 Genetic Analyzer). The analysis determined that the patient was homozygous for a new c.1182del mutation (deletion of a single nucleotide) in exon 7 of the alpha-GLA A gene. The patient's mother and sister were investigated and found to be negative for this mutation. The patient's remaining siblings were not available for the study, but none of them had manifested clinical symptoms associated with the disease, to date. The patient had a son, and so the mutation was not transmitted to his offspring. Hormone replacement therapy was started with agalsidase alfa (Replagal®). During follow-up, his oedema and neuropathic pain improved, but his renal function progressively worsened and became end-stage renal disease, in a period of 3 years. The patient started haemodialysis, and one year later, he received a living-donor kidney graft from his wife. The patient, during post-transplant follow-up, maintained stable renal function with an estimated glomerular filtration rate of 45ml/min/1.73m2 and proteinuria levels of 200mg/24h. He underwent follow-up biopsies at 3 months and one year that showed no Gb3 deposits in the renal tissue. Regarding his cardiac progression, clinically he had the onset of dyspnoea on exertion, in addition to a worsening of his myocardial fibrosis, assessed by cardiac magnetic resonance imaging in February 2014. It was decided to change his treatment to agalsidase beta (Fabrazyme®) from then onwards.
DiscussionParapelvic cysts are not uncommon in FD, but they are not specific to this condition. In a previous study of 122 FD patients (76 men and 40 women), it was observed an increased frequency of cortical and parapelvic cysts as compared to the general population.6 Another study found that parapelvic renal cysts were more common in patients with FD than in healthy control subjects.7 Although our patient did not show angiokeratomas, one of the most common clinical manifestations in patients with FD, his signs and symptoms and the findings in his imaging studies led us to suspect a genetic basis for the disease. The finding of multiple cysts in the renal sinus in a young patient with kidney failure of unknown origin should raise the possibility of FD in the appropriate clinical context.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Azancot MA, Vila J, Domínguez C, Serres X, Espinel E. Múltiples quistes parapiélicos en la enfermedad de Fabry. Nefrologia. 2016;36:310–312.