Objetivo: Análisis de la aplicación de un protocolo multidisciplinar para el mantenimiento de las fístulas arteriovenosas para hemodiálisis protésicas. Método: Recogida prospectiva de todas las intervenciones realizadas para el tratamiento de la disfunción de las fístulas arteriovenosas protésicas (FAVP) en el período 1999-2007 siguiendo un protocolo multidisciplinario. Las estenosis se trataron mediante angioplastia, (ATP) excepto en casos de persistencia o recurrencia antes de tres meses. Las trombosis fueron tratadas siempre mediante trombectomía quirúrgica y puente de PTFE si fue necesario. Se analizan el número y el tipo de procedimientos, las complicaciones, la permeabilidad primaria y secundaria de las FAVP. Resultados: Se completó el seguimiento de 96 FAVP. Todas fueron prótesis de PTFE de 6x40 mm (Gore-Tex®). Treinta y seis se colocaron en el antebrazo con anastomosis humerobasílica en asa y 60 en el brazo con anastomosis humeroaxilar curva. Durante el período de estudio fueron necesarios 131 angioplastias transluminales percutáneas, 15 stents, 109 trombectomías y 52 puentes a vena proximal para el mantenimiento de la permeabilidad de las FAVP. La permeabilidad primaria fue del 73,68 %, 60,21 % y 37,52 % a 1, 2 y 3 años, respectivamente. La permeabilidad secundaria fue del 89,49 %, 84,07 % y 66,84 % a 1, 2 y 3 años, respectivamente. Se consiguió evitar la colocación de un catéter central en el 80 % de las intervenciones por trombosis. No se produjeron muertes relacionadas con los procedimientos. La tasa de ingresos hospitalarios relacionados con la trombosis de las FAVP fue de 0,03 paciente/año. Conclusiones: La aplicación de un protocolo multidisciplinar en el tratamiento de las disfunciones de las FAVP siguiendo las recomendaciones de las guías internacionales prolonga la permeabilidad de las FAVP y disminuye el uso de catéteres centrales.
Purpose: To analize the clinic results of the implantation of a multidisciplinary protocol to maintain ermeability of the arteriovenous hemodialysis grafts (AVG). Methods: Prospective recording of all interventions (radiological and surgical) on AVG dysfunction in the 1999-2007 period. The AVG stenosis were always treated by percutaneous angioplasty (PA) except stenosis recurrence in less than three months or persistence after PA. The AVG thromboses were always treated by surgical thrombectomy plus PTFE bridge if necessary. Complications, primary and secondary AVG patency were reviewed. Results: Ninety six dysfunction AVG were collected for study. All of them were 6x40mm standard wall PTFE (Gore-Tex®). Thirty six of them were humero-basilic antebraquial loop and sixty were humero-axillary upper arm curve configuration. During the study, 52 PTFE bridges, 109 surgical thrombectomies, 131 PA, and 15 stents were needed to maintain FAVP permeability. Primay patency was 73.68%, 60.21% and 37.52% at one, two and three years respectively. Secondary patency was 89.49%, 84.07% and 66.84% at one, two and three years respectively. We avoid a central venous catheter (CVC) in the 80% of intervention for thrombosis dysfunction. No surgical or radiological related deaths occurred. Median hospital admission related with AVG thrombosis was 0.03/patient/year. Conclusion: The application of a combined protocol for the treatment of AVG dysfunction and thrombosis, according to DOQI recomendations obtains good results in AVG patency in our experience.
INTRODUCTION
In Spain, the incidence and prevalence of chronic kidney disease patients who require renal replacement therapy have increased more than 100% over the last 15 years1 (from 61 and 392 patients per million in 1991 to 132 and 1009, respectively, in 2007). The age group with the highest percentage increase is patients over 75 years of age (from 8.5% of the patients prevalent in 1992 to 40% currently). In this group, most patients are treated by haemodialysis (94% of incident patients) and few change technique throughout their lives. Approximately 10% of haemodialysis patients in our setting are treated with prosthetic arteriovenous fistulas (AVG).2-5
AVG are a good option in patients whose superficial venous system is inadequate for creating an autologous fistula or fistulas after previous loss of fistulas. They have the advantage of being easy to cannulate, their early failure rate is low and they have a short maturation time. However, their complication rate is much higher than that of native arteriovenous fistulas (n-AVF) and their long-term patency is lower. The treatment of AVG stenoses and thromboses can be performed by radiological or surgical methods. Each centre should establish maintenance protocols in accordance with their resources in order to achieve healthcare quality indicators that comply with international recommendations.6,7
In our hospital, we have a multidisciplinary haemodialysis vascular access team that has taken a particular interest in developing protocols and defining indicators in our setting.8,9
In this study, we present our AVG maintenance protocol and the evaluation of its results after eight years of follow-up.
MATERIAL AND METHOD
Prospective study on the application of a protocol for the treatment of AVG dysfunction (stenosis and thrombosis).
Setting
Our hospital provides haemodialysis vascular access service for a healthcare catchment area in the Community of Madrid of as well as regularly serving the units of the provinces of Ávila and Segovia (250 000 inhabitants). Here, the medical records are computerised and there is a specific protocol for interventions related to arteriovenous fistulas that the head surgeon completes at the end of intervention. The interventions were performed by four surgeons of the service involved but not exclusively dedicated to it (their activity is that of any general surgeon) without specific shifts. There is no urgent service for radiological procedures.
Patients
During the 1999-2007 period, 115 patients received 130 AVG (15% of the total arteriovenous fistulas performed). We excluded 34 AVG patients from the study due to a lack of follow-up (early failure or transfer to other centres). In the rest (96), a multidisciplinary protocol was applied for the diagnosis and treatment of dysfunctions and thrombosis. In the analysis of results, we were assisted by computerised medical records and a specific surgical protocol in the case of vascular access for haemodialysis.
Surgical technique
It was decided to introduce a AVG after ruling out the possibility of creating an autologous fistula because of an inadequate superficial venous system or after failure of previous fistulas. The material used in all cases was expanded polytetrafluoroethylene (ePTFE 6mmx40cm). The anatomical configuration of the AVG was decided on the basis of the calibre of the patient’s deep venous system, using two approaches:
Follow-up
A fistulography was requested in cases of dysfunction of the AVG with dysfunction being considered as a dynamic venous pressure >200mm/Hg with flows of 300ml/min, Kt/V <0.8 or recirculation >25%).
Protocol
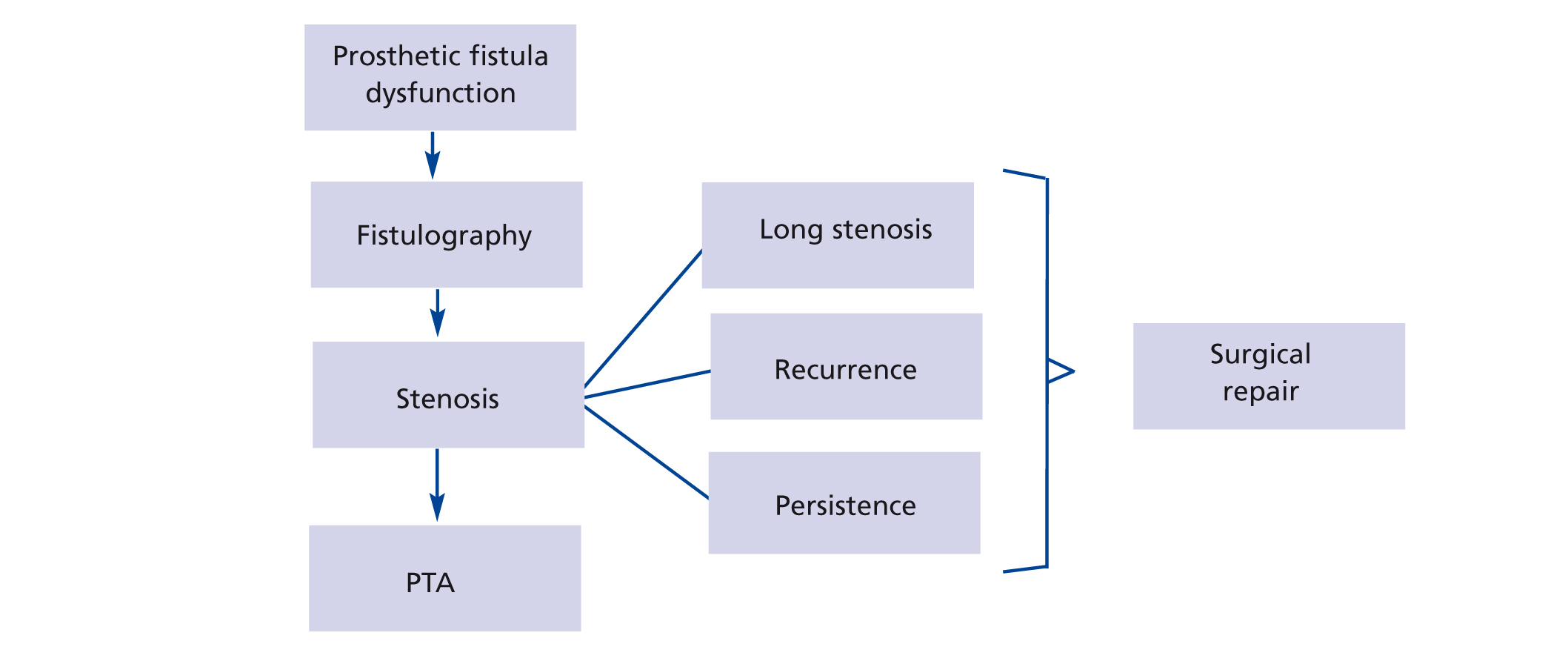
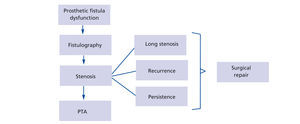
The preferred initial treatment in the case of stenoses (Figure 1) of the AVG was percutaneous transluminal angioplasty (PTA) conducted by interventional radiologists, except in the case of multiple or long stenoses or complete obstruction of the drainage vein. The latter was carried out by antegrade puncture of the prosthetic fistula. After studying the Doppler echogram and fistulogram, the stenoses received a 0.035” Terumo hydrophilic guidewire, with a 6F feeder being positioned. The PTA indication was based on a morphological study of the stenoses, with pressure gradients only being measured in uncertain cases. The balloon diameter was 6mm for intraprosthetic stenoses, with diameters of 7mm to 8mm at the anastomoses or at native vein level. In the case of resistant or recurrent stenosis, cutting balloon catheters were used (over a 0.014” guidewire), which were of a similar calibre to the conventional balloons. A stent was introduced in cases of central stenosis whose surgical repair would require very invasive surgery.
Indications for surgery on stenoses are limited to persistent stenoses (a suboptimal result of percutaneous dilation) or stenoses recurring in less than three months, with an analysis of the stenotic area being performed in these cases by the surgical technique necessary in each case (in the case of stenosis in the venous anastomosis, proximal vein bypass was performed; if the stenosis was intraprosthetic, the prosthesis was partially replaced; and if the stenosis was found at the arterial anastomosis, a new proximal anastomosis was created provided there had been no stenosis in the distal artery [due to the risk of aggravating the ischaemia]).
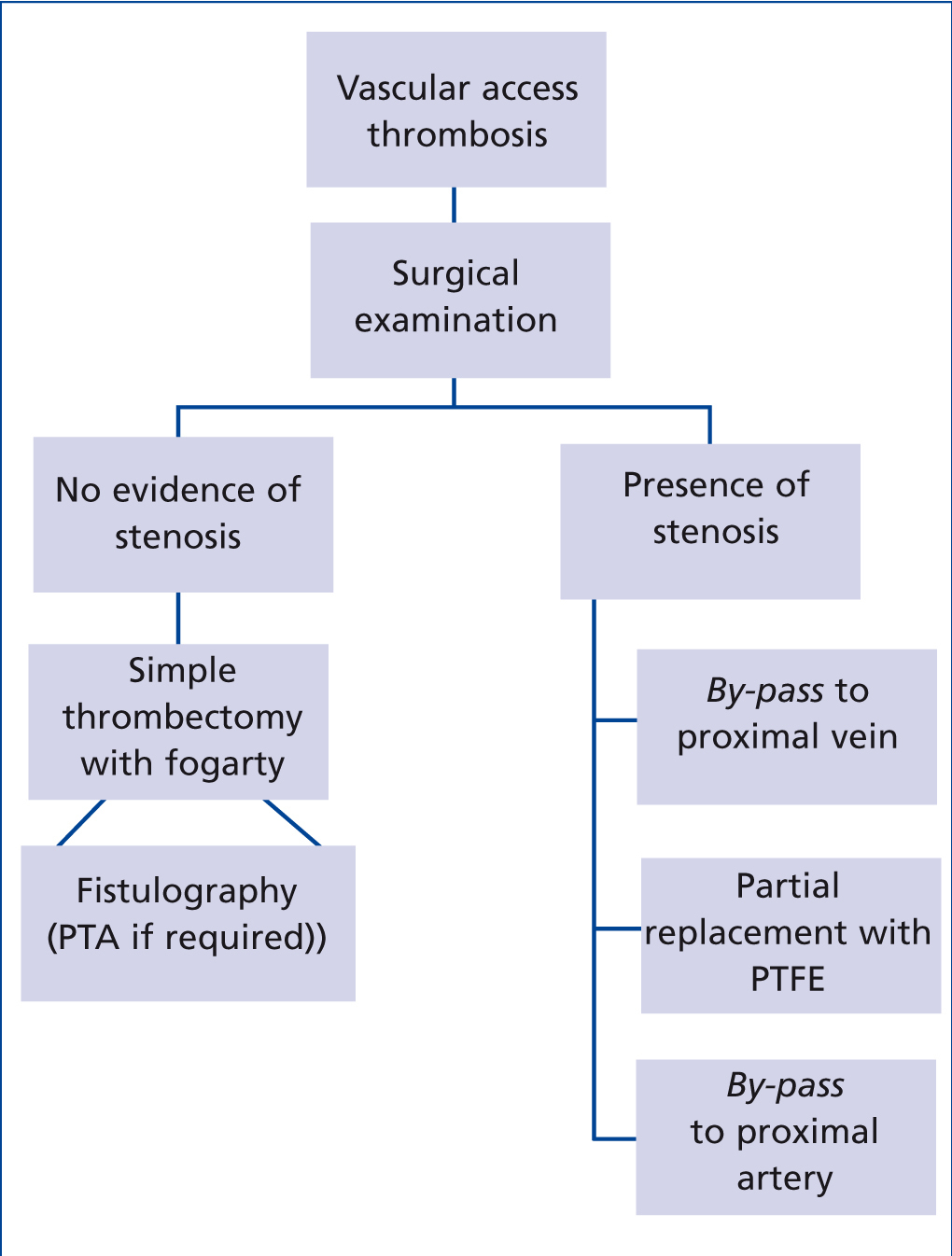
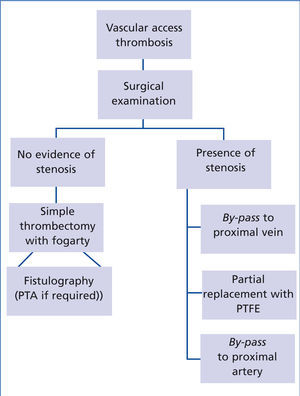
Thromboses (Figure 2) were treated urgently in order to avoid the use of unnecessary catheters. Surgical thrombectomy was carried out with a Fogarty catheter. If the surgeon did not find a reason for the thrombosis, an urgent fistulography was carried out and the stenoses detected by PTA were treated. If the surgeon found a reason for the latter, it was corrected by a prosthetic bridge in the stenotic area.
Definitions and statistical analysis
Primary survival: time until the first thrombosis of AVG.
Secondary survival: time until the definitive thrombosis.
Thrombosis rate: episodes of thrombosis/AVG at risk/year.
Number of admissions related to AVG thrombosis.
After surgical repair due to thrombosis, the AVG was considered to be patent if it could be used in the next dialysis session, avoiding a central venous catheterisation (CVC).
Statistical analysis was carried out with the SPSS software using Kaplan-Meier survival curves and carrying out comparison by the log-rank test.
No patient received antiplatelet or anticoagulant treatment as an indication for maintaining the patency of the vascular access.
RESULTS
A total of 96 AVG in 88 patients were included in the study and follow-up using the protocol. Thirty-six of the AVG were LOOP and 60 were HAX.
During the study period, in accordance with the protocol, 131 PTA, 15 stents, 109 thombectomies and 52 proximal vein bridges were necessary to maintain the patency of the AVG.
There were no procedure-related deaths (either in the incorporation of the AVG or in their repair) and there was only one infection after stenting, requiring the removal of the stent. There were no pulmonary embolisms, including in the humeral artery. The patency rate after surgical treatment of a thrombosis was 80%, with a CVC being avoided in 80% of the thrombosis episodes.
At the end of the study 63.5% (n=61) of AVG were patent and 36.4% (n=35) of fistulas were thrombosed. 38.5% (n=37) of the fistulas remained patent when the patient died. 5.1% (n=5) of patients were transplanted with patent AVG. At the end of follow-up, 19.7% (n=19) remained patent and patients were on a haemodialysis programme.
The annual rate of AVG thrombosis-related hospital admissions was 0.03 admissions/patient/year.
The annual thrombosis rate for AVG during this period was 0.45.
Primary patency
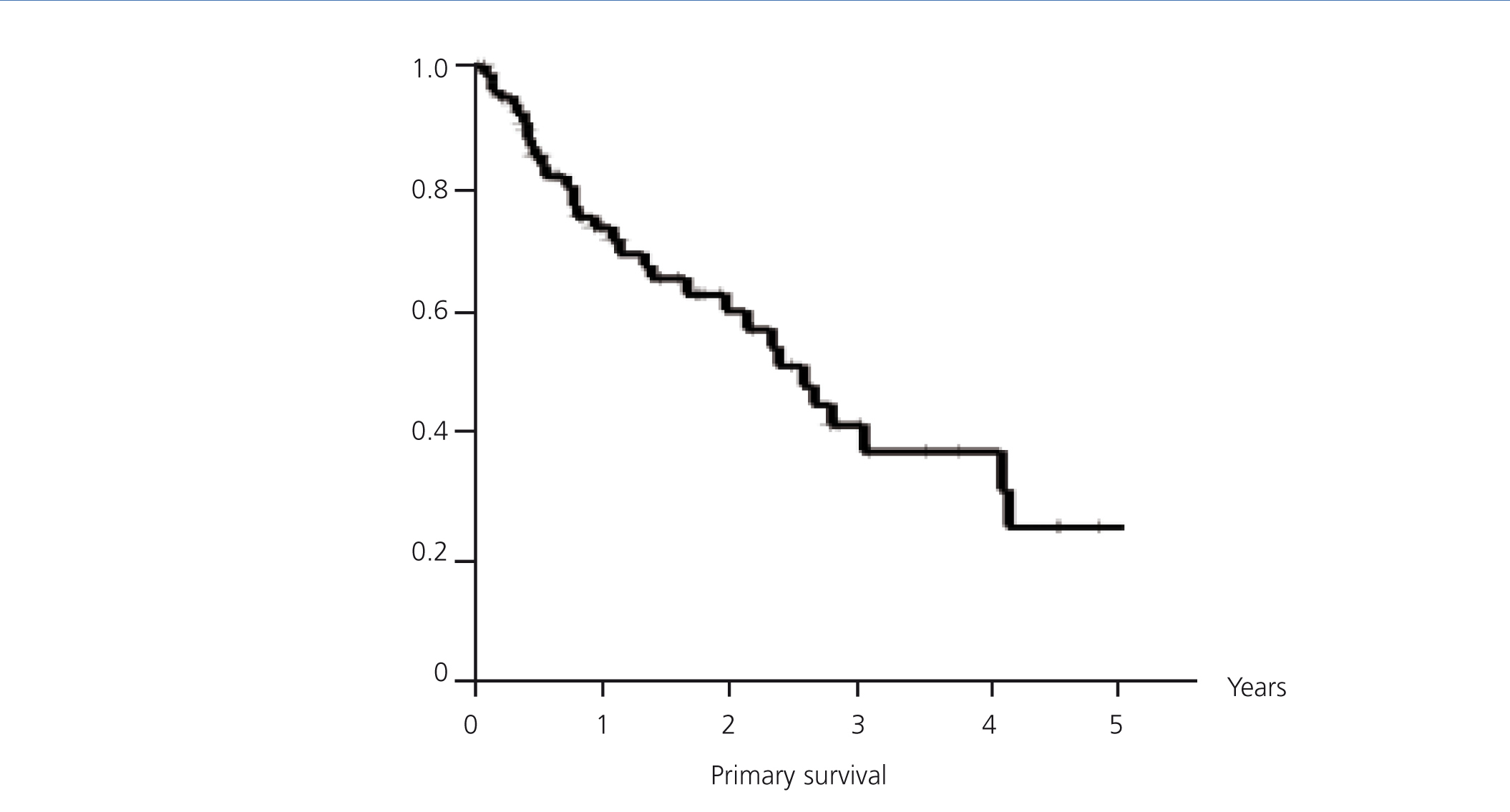
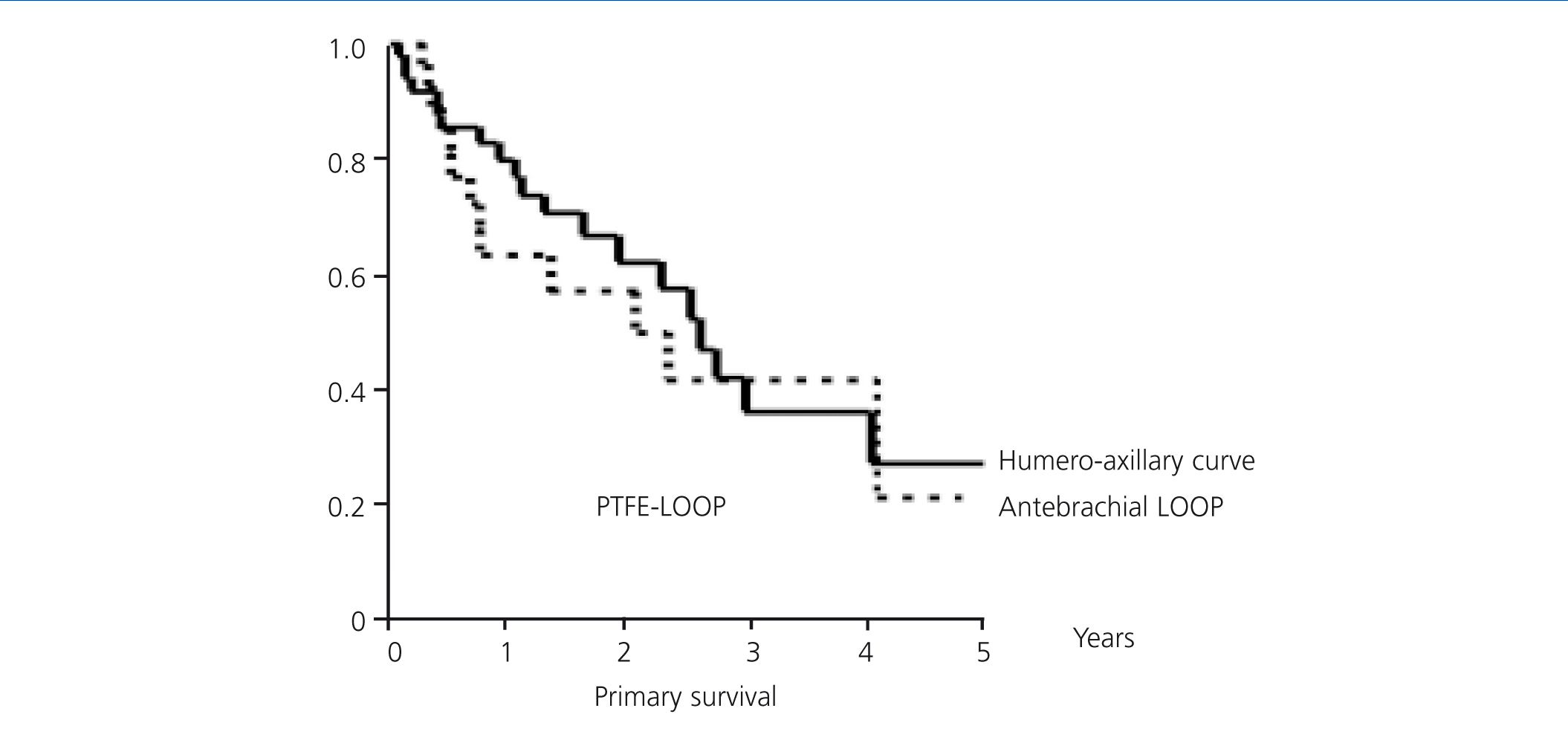
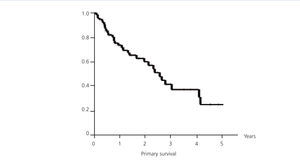
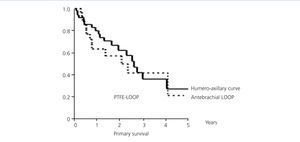
The overall mean primary patency (Figure 3) was 2.55 years, with a confidence interval (CI) of 95% (1.91-3.17). One year survival was 73.68%, at two years, it was 60.21% and at three years, it was 37.52%. A LOOP type fistula had a mean survival of 2.1 years (0.65 to 3.55). One year survival was 62.99%, at two years it was 56.7% and at three years, it was 41.34%. A HAX type fistula had a median survival rate of 2.6 years (2.02 to 3.23). One year survival was 79.9%, at two years it was 61.96%, and at three years it was 41.59% (log-rank 0.33, p-value .57) (Figure 4).
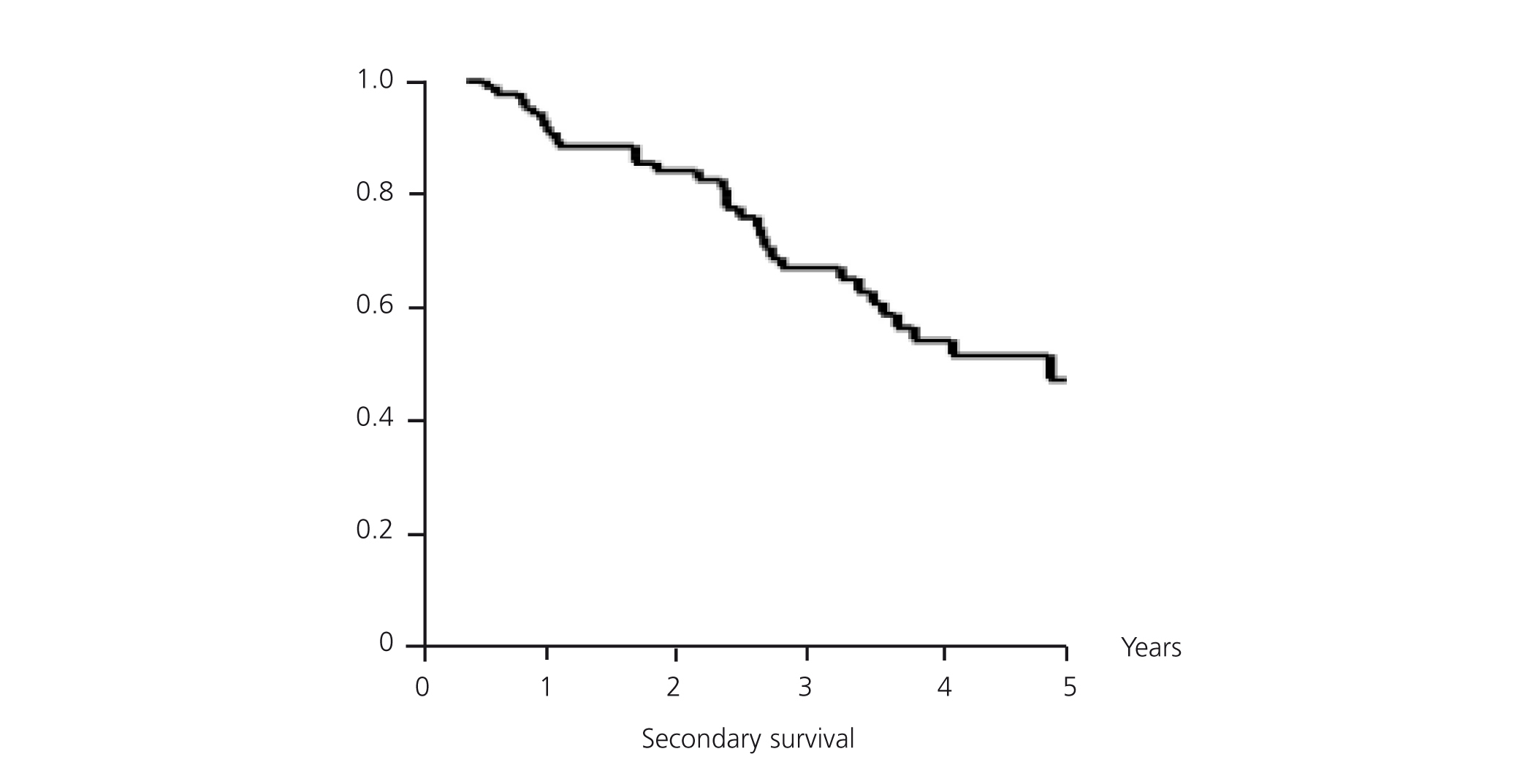
Secondary patency
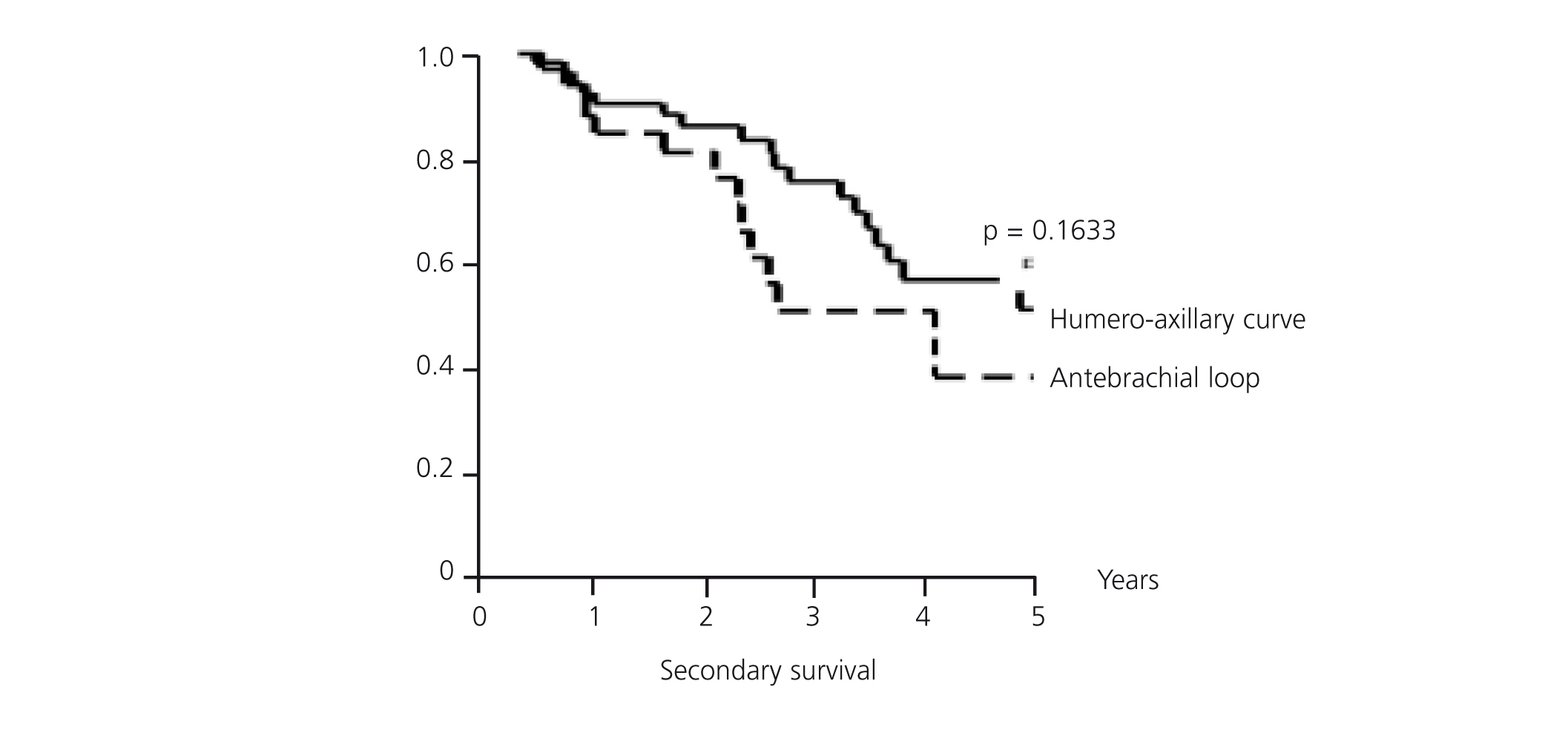
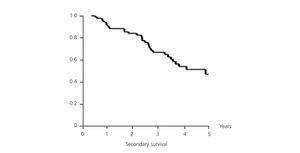
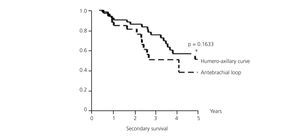
Mean secondary survival was 4.65 years, with a CI of 95% (3.96-5.34). At one year, the fistula survival rate was 89.49%, the two-year survival rate was 84.07% and at three years 66.84% survived (Figure 5). The LOOP type fistulas had a mean survival of 4.13 years (1.84-6.43). At one year 87.88% survived, at two years 81.06% survived, and at three years the survival rate was 50.66%. The HAX type fistulas have a mean survival of 5.71 years (2.63-8.79). At one year, 90.54% survived, at two years 86.07% and at three years, 75.46% (log-rank 1.94; P-value .1633) (Figure 6). Although the patency of HAX was greater than that of LOOP, there are no statistically significant differences.
DISCUSSION
In spite of displaying a higher rate of complications than n-AVF, AVG may be an excellent solution for patients with an exhausted superficial venous system and in our experience, they may also be the definitive access in elderly patients (almost 40% of our patients died with patent AVG). Surgery is not much more complex than for an n-AVF and it can be performed under local anaesthetic on an outpatient basis.10 Furthermore, if maintenance protocol is applied, it is possible to rescue most AVG, achieving patency rates of 90% at one year and over 50% at three years.10-12
In our centre, there has been a multidisciplinary group of specialists in haemodialysis vascular access for 10 years made up of nephrologists, radiologists and surgeons. They have designed follow-up protocol and healthcare quality indicators.8,9 Furthermore, a distribution of work has been carried out amongst the services involved:
- The clinical follow-up of AVG, as well as the diagnosis of dysfunctions, is carried out by the Nephrology Service and a special effort has been made to detect dysfunctions at an early stage.
- Treatment of dysfunctions (both stenosis and thrombosis) of the AVG may be carried out by radiological or surgical methods. In general, the advantage of radiological techniques compared to surgical techniques is the “saving” of proximal vascular territory and its disadvantage is the requirement for a greater number of procedures to maintain AVG patency.13-21
The protocol designed in our centre is based on the DOQI recommendations and adapted to the availability of our means. It was never our aim to compare techniques, but rather to apply the most appropriate technique in each case, in accordance with the means available and the experience of the doctors.
Treatment of thrombosis has been considered an urgent procedure to avoid CVC. The availability of the vascular radiology room in our centre is limited, and urgent procedures are not able to be performed; therefore, the treatment of thrombosis was always carried out by the Surgery Service, due to the availability of a 24-hour service. The diagnosis and treatment of stenoses was in most cases carried out on a scheduled basis by the Radiology Service. This approach has the advantage of performing the diagnosis and treatment of dysfunction in the same process, reserving surgery for cases of persistence or relapse.
In our experience, the rigorous application of this protocol allows healthcare quality indicators to be maintained, in accordance with those who demand national and international guidelines6,7 for the treatment of AVG dysfunctions and thrombosis: patent percentage at three years above 50% and a thrombosis rate below 0.5 episodes per patient and year.
These data have repercussions on other healthcare indicators, since AVF are a major source of morbidity and hospital admissions. Thus, in our centre, the complications of vascular access are not the main reason for admission, with 0.1 episodes per patient and year,9 that is, half of the figures published in other series,3 and specifically, there are minimal hospital admissions related to thrombosis of AVG.
We firmly believe that the best results in the maintenance of the AVG are obtained with a multidisciplinary approach.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Protocol for prosthetic arteriovenous fistula dysfunction due to stenosis
Figure 2. Protocol for thrombosis of the prosthetic arteriovenous fistula
Figure 3. Primary survival (overall)
Figure 4. Primary survival of antebrachial LOOP/HAX
Figure 5. Secondary survival (overall)
Figure 6. Secondary survival of antebrachial LOOP/HAX