The increase in demand for medical care for renal complications associated with neoplastic diseases is a reality in most nephrology departments. In response to this overall situation, the creation of healthcare models such as monographic consultations and develop training programs in Onconephrology could improve the care of these patients.
Through an exploratory and descriptive study, we identified current situation of kidney involvement in cancer patients. The objective of the present study is to establish the criteria for specific assistance in the field of Onconephrology. For this, we have reviewed key aspects and analyzed the current situation in our country, through a survey addressed to all nephrologists through the Spanish Society of Nephrology., together with the experience of two Spanish centers. From this information, we have established some requirements and recommendations for the start-up of these consultations.
El incremento de la demanda asistencial de patología renal asociada a enfermedades neoplásicas es una realidad en la mayoría de servicios de nefrología. Para dar respuesta a esta situación, debe considerarse la creación de modelos asistenciales como consultas monográficas y desarrollar programas de formación en Onconefrologia que permitan optimizar la atención de estos pacientes.
A través de un estudio exploratorio y descriptivo, identificamos cual es la situación actual de la afectación renal en pacientes con cáncer. El objetivo del presente estudio es establecer los criterios para la asistencia específica en el ámbito de la Onconefrologia. Para ello hemos revisado aspectos clave y analizado la situación actual en nuestro entorno, mediante una encuesta dirigida a todos nefrólogos a través de la S.E.N., junto a la experiencia de dos centros españoles. A partir de esta información hemos establecido una serie de requisitos y recomendaciones para la puesta en marcha de estas consultas.
In recent years, nephrology has been witnessing an increase in the care needs of patients with oncologic disease.1,2 For this reason, both nephrologists and oncologists believe that collaboration is needed to enable the optim[isation of care for these patients. Onconephrology is emerging as an increasingly complicated and rapidly growing field of medicine. The reasons for this are the higher prevalence of kidney complications in patients with cancer and also from the prevalence of cancer in patients with kidney disease.
In kidney disease associated with cancer is predominant the possibility of acute kidney injury (AKI), which is sometimes associated with nephrotoxic drugs3–6 and increases dramatically in the presence of pre-existing chronic kidney disease (CKD). Furthermore, some types of cancer and cancer treatment may also have deleterious effects on glomerular structure and cause proteinuria by damaging podocytes.7 The connection between cancer and the kidneys is broadened with kidney damage related to immunotherapy, electrolyte disorders, problems linked to chemotherapy doses and timing in patients with CKD on dialysis, partial or total nephrectomy in patients with cancer and CKD, and matters related to kidney transplantation (donors and recipients with a history of cancer).8
Onconephrology is already an established subspeciality in many hospitals. Many publications and international organisations, such as Kidney Disease Improving Global Outcomes (KDIGO), have suggested that this special care should be organised at leading oncology and nephrology centres.1,9–13 However, at present, there is no extensive, verified data on certain aspects such as the extent to which such practices would be frequented.
Some studies have already found that patients with cancer have a risk of AKI at one year of 17.5% and at five years of 27%,3 and a prevalence of stage 3 CKD of 12%.5 The impact on mortality is also noteworthy. The lower the glomerular filtration rate, the higher the mortality among patients with cancer.14
In addition, some studies have found that the lower the glomerular filtration rate, the higher the risk of onset of cancer. In general, a decrease of 10 ml/min in glomerular filtration rate increases the risk of cancer by 29%.15 In patients with renal replacement therapy on haemodialysis, the cumulative risk of developing tumours at five years has been estimated at 9.5%.16 Special mention must be made of the fact that patients who undergo kidney transplantation have a 2.1 times higher likelihood than the general population of developing cancer.17
The risk of AKI in patients with cancer during hospitalisation has also been demonstrated in several studies, with a longer hospital stay and a higher risk of complications also observed.1,18
The complexity of the relationship between the two types of disease (cancer and kidney disease) is increasingly evident and common, rendering a multidisciplinary approach essential for suitable and optimal management of the disease, as well as its prevention, treatment and complications. In addition, training of subspecialists in onconephrology and the establishment of dedicated practices should represent the initial point of support in early, comprehensive assessment of this patient group, both to prevent major complications and unnecessary hospital admissions and to develop areas of research among the different specialities in onco-haematology and onconephrology.
This study reviews the reasons for referring these patients, the characteristics of the multidisciplinary approach, the assessment of onconephrology patients and the situation of onconephrology practice in Spain. It also presents the limited experience to date in Spain with this type of practice, with a view to raising its profile, and identifies some requirements for its implementation.
Added value of monographic outpatient clinics monIn recent years, due to changes in various specialisations and in response to growing care needs, efforts to establish specialized dedicated centres have become more consolidated. In general, their objective has been to improve health results through, among other things, a cross-cutting approach to the disease in question, a decrease in clinical variability, and a better quality of care for patients with low-prevalence diseases, or in clinical situations that in many cases are more complex.
Specialities such as cardiology, pneumology and rheumatology have been implementing practices with these characteristics for the care of patients with specific diseases. Heart failure, chronic obstructive pulmonary disease, home oxygen therapy and arthritis are some examples of practices that have demonstrated their usefulness in the form of decreased numbers of hospital admissions and emergency episodes.19–23
Within nephrology itself, the development of advanced CKD units, as an example of dedicated practices, has been taking on more and more importance in nephrology services. The Sociedad Española de Nefrología [Spanish Society of Nephrology] recently presented the ACERCA project as a model for quality accreditation of these units.24 Unified results may be obtained based on the use of common standards. Up to now, unfortunately, objective data on this type of practice are not available, despite the time since establishment thereof at many hospitals in Spain.
In addition, within nephrology there is some other multidisciplinary experience, as in the case of patients with heart disease and kidney disease. These are complex patients whose concomitant heart failure and CKD benefit from a joint approach to treatment by both cardiologists and nephrologists. This practice may also be used in screening for heart disease with a view to kidney transplantation, with benefits reflected in lower mortality.25
The creation of onconephrology monographic consultations could therefore represent a clear benefit for patients in whom the approach to concomitant cancer and kidney disease proves complex. The possibilities of managing specific cases and quickly and efficiently contacting the referral onconephrology team would facilitate decision-making in this population and are viewed positively by the other specialisations involved.
Key considerations in setting up an onconephrology monographic consultationCriteria for referralTable 1 shows the main reasons for referral to an onconephrology practice. AKI is common in patients with cancer.26,27 According to a Danish study conducted in 2011, which analysed 37,267 incident patients with cancer, the risk of presenting AKI was 17.5% at one year and 27% at five years — much higher than that observed in the general population.3
Main reasons for referral to an onconephrology outpatient clinic.
| Renal insufficiency (before, during or after cancer diagnosis/cancer treatment) |
| HTN of recent diagnosis/poor blood pressure management in a patient with prior HTN during cancer treatment |
| Electrolyte disorders |
| Proteinuria/sediment abnormalities |
| Assessment of patients with a diagnosis of kidney cancer |
| Planning of surgical strategy and (neo)adjuvant treatments in patients with CKD and kidney cancer |
| Assessment of risk of progression of CKD in patients who are to undergo nephrectomy |
| Cancer and kidney transplant |
| Assessment of inclusion on transplant waitlist in patients with a history of cancer |
| Cancer screening in patients with kidney transplant |
| Management of immunosuppression in patients with kidney transplant |
| Haematological disease and kidney impairment |
| Plasma cell myeloma |
| Amyloidosis |
| MGRS |
| Chronic lymphocytic leukaemia |
| Lymphoma |
| Assessment of RRT in patients with CKD |
| Initiation of RRT in patients with active cancer |
| Suspension of RRT in patients with advanced cancer |
CKD: chronic kidney disease; HTN: hypertension; MGRS: monoclonal gammopathy of renal significance; RRT: renal replacement therapy.
AKI represents a major negative impact on morbidity and mortality for these patients: it increases the risk of chemotherapy toxicity, jeopardises the continuity of effective treatments, limits patient participation in clinical trials and requires doses of highly effective drugs to be decreased or less effective alternative treatments to be sought.4
Relatively often, kidney function will deteriorate regardless of the course of a cancer or its treatment, although in many cases it may be promoted by these things, as this population is more susceptible to functional factors and to the kidney toxicity of commonly used drugs such as diuretics and non-steroidal anti-inflammatory drugs.
However, these patients may also present kidney damage directly related to neoplastic disease or the treatment received.7,28,29 Nephrologists should be familiar with the diagnosis and management of diseases such as tumour lysis syndrome and cast nephropathy (myeloma kidney), and with toxicities resulting from an oncology treatment arsenal constantly in development. This requires constant updating so that early identification and suitable treatment are possible. Table 2 summarises the main causes of AKI in patients with cancer.
Acute kidney injury in oncology patients.
| Prerenal | Low intake, vomiting, diarrhoea, sepsis, anaemiaHypercalcaemiaDrugs (diuretics, NSAIDs, ACE inhibitors/ARBs, calcineurin inhibitors) |
|---|---|
| Postrenal or obstructive | Urinary tumours |
| Extrinsic compression due to lymphadenopathy, abdominopelvic tumours | |
| Retroperitoneal fibrosis (history of prior radiotherapy) | |
| Parenchymal | Tubulointerstitial |
| Toxic ATN due to intravenous contrast | |
| Toxic ATN due to other drugs (NSAIDs, aminoglycosides, amphotericin B, aciclovir, calcineurin inhibitors) | |
| Tubular toxicity due to platin or crystals (methotrexate) | |
| ATIN due to drugs (NSAIDs, antibiotics, PPIs, allopurinol, etc.) | |
| Immune-mediated interstitial nephritis associated with checkpoint inhibitors | |
| Tumour lysis syndrome | |
| Cast nephropathy (myeloma kidney) | |
| Tumour infiltration | |
| Sinusoidal obstruction syndrome (hepatic veno-occlusive disease) | |
| Glomerular | |
| Paraneoplastic glomerulonephritis | |
| Podocyte damage due to drugs (TKIs, anti-VEGF therapy, mTor inhibitors, biphosphonates, interferon) | |
| Immune-mediated glomerulonephritis associated with checkpoint inhibitors | |
| MGRS (monoclonal immunoglobulin deposition disease, AL amyloidosis, glomerulonephritis with a membranoproliferative pattern) | |
| Vascular | |
| Paraneoplastic TMA, GVHD, nephropathy due to radiation, nephropathy due to drugs (anti-VEGF therapy, TKIs, gemcitabine, mitomycin C, checkpoint inhibitors) |
ACE inhibitors: angiotensin converting enzyme inhibitors; ARBs: angiotensin II receptor blockers; ATIN: acute tubulointerstitial nephropathy; ATN: acute tubular necrosis; GVHD: graft versus host disease; NSAIDs: non-steroidal anti-inflammatory drugs; PPIs: proton pump inhibitors; TKIs: tyrosine kinase inhibitors; TMA: thrombotic microangiopathy; VEGF: vascular endothelial growth factor.
It must not be forgotten that cancer patients are often subject to radiological examinations with intravenous contrast. This, associated with risk situations such as volume depletion and comorbidities such as diabetes mellitus and CKD, renders these patients particularly vulnerable to the development of post-contrast AKI as well as gradual loss of renal functional reserve.30 The use of iso-osmolar contrasts and the implementation of prevention protocols may be useful for minimising the risk of nephrotoxicity. The objective is to ensure proper intravenous hydration and suspension of medications with repercussions for volaemia or intrarenal flow regulation mechanisms (renin-angiotensin-aldosterone system blockade, non-steroidal anti-inflammatory drugs or fibrates).
Another one of the most common reasons for a consultation is CKD, which has a high prevalence in the population with neoplastic disease.14,17 Estimated glomerular filtration rate is very important for proper adjustment of doses of drugs that may be nephrotoxic, and for continuing or not continuing cancer treatment that may determine the patient's survival. The search for the best method for estimating glomerular filtration rate in the general population remains controversial issue and is even more discussed in patients with cancer.31,32 CKD-EPI adjusted to body surface area appears to show a good correlation to actual glomerular filtration rate.33 Other studies have shown that equations based on cystatin may offer a benefit over those based on creatinine.34 Exact measurement using nuclear medicine techniques (scintigraphy with technetium-99 m DTPA) at experienced centres is the gold-standard technique. In some cases, other functional tests (within the study of obstructive uropathy) and/or a kidney biopsy must be performed to arrive at a definitive diagnosis. This is sometimes extremely important due to, for example, implications for continuing an effective cancer treatment.
Electrolyte disorders, which are also common, as occurs with AKI, may be due to causes similar to those identified in the general population (hyponatraemia associated with the use of thiazides or hypokalaemia due to gastrointestinal losses) or may be associated with cancer (e.g. paraneoplastic syndrome of inappropriate antidiuretic hormone secretion) or chemotherapy (e.g. hypomagnesaemia in patients treated with platins or cetuximab).35Table 3 shows the main electrolyte imbalances in cancer patients.
Main electrolyte imbalances in cancer patients.
| Hyponatraemia | Gastrointestinal losses, thiazides, inadequate fluid therapy |
| CHF, cirrhosis, AKI | |
| Hypothyroidism, adrenal insufficiency | |
| Paraneoplastic SIADH (small cell lung carcinoma, head and neck cancers, brain cancers, etc.) | |
| SIADH due to drugs (cyclophosphamide, vincristine, vinblastine, cisplatin) | |
| SIADH due to pain, nausea/vomiting | |
| Hypokalaemia | Diuretics, gastrointestinal losses, postobstructive polyuria |
| Cisplatin, ifosfamide, amphotericin B, aminoglycosides | |
| Ectopic ACTH secretion (bronchial carcinoid tumours, small cell carcinoma and adenocarcinoma of the lung) | |
| Hyperkalaemia | AKI |
| Adrenal insufficiency | |
| Tumour lysis syndrome | |
| Calcineurin inhibitors, NSAIDs, trimethoprim/sulfamethoxazole | |
| Hypophosphataemia | Cachexia, malnutrition, vitamin D deficiency |
| Platins | |
| Fanconi syndrome (multiple myeloma) | |
| Oncogenic osteomalacia (chondrosarcoma, osteoblastoma, haemangiopericytoma) | |
| TKIs | |
| Hypercalcemia | Secretion of PTHrP by the tumour (epidermoid carcinoma of the lung, cervix or stomach) |
| Osteolysis (multiple myeloma, bone metastasis of breast or lung carcinoma) | |
| Production of 125-OH-vitamin D (lymphomas) | |
| Hypovolaemia | |
| Hypomagnesaemia | Platins, cetuximab, panitumumab |
| Loop diuretics, proton pump inhibitors |
ACTH: adrenocorticotropic hormone; AKI: acute kidney injury; CHF: congestive heart failure; NSAIDs: non-steroidal anti-inflammatory drugs; PTHrP: parathyroid hormone-related protein; SIADH: syndrome of inappropriate antidiuretic hormone secretion; TKIs: tyrosine kinase inhibitors.
Assessment of patients with haematologic disease who present deterioration of kidney function or proteinuria represents a significant part of the work of onconephrology practices. In multiple myeloma, kidney impairment in the form of cast nephropathy requires production of large amounts of paraprotein (this being considered a defining event of multiple myeloma). However, in the two other most common forms of kidney impairment associated with dysproteinaemias (AL amyloidosis and Randall-type monoclonal immunoglobulin deposition disease), the paraprotein's physicochemical properties, not its amount, are what determine kidney injury. Therefore, it is uncommon for these patients to meet haematologic criteria for chemotherapy, the initiation of which is determined by kidney impairment.36 To assess them, electrophoresis and immunofixation in blood and urine, as well as free light chains in blood, must be ordered. Most cases require histological diagnosis via kidney biopsy (including immunofluorescence and electron microscopy techniques) to determine the nature of the injury. This enables assessment of the form of expression within the term monoclonal gammopathy of renal significance, its severity and the need for treatment of the haematological disease.
Table 4 shows particular considerations in assessment of onconephrology patients to be conducted at an onconephrology practice for a comprehensive approach.
Patient assessment at an onconephrology practice.
| Medical history | Personal and family history of kidney disease |
| Comorbidities associated with kidney disease (HTN, T2D, etc.) | |
| Taking of nephrotoxic drugs | |
| Recent examinations with intravenous contrast media | |
| Tumour type and staging | |
| Cancer treatment lines received | |
| Current stage of cancer, active treatment, response to treatment, prognosis | |
| Physical examination | Blood pressure |
| Volume status assesment | |
| Laboratory tests | Clinical chemistry, complete blood count, venous blood gas, urinalysis and urine sediment. Urine protein/creatinine ratio |
| Assessment of glomerular filtration rate (CKD-EPI adjusted for BSA, nuclear medicine) | |
| Electrophoresis and immunofixation in blood and urine, free light chains in blood (patients with monoclonal gammopathy) | |
| Imaging tests | |
| Other | AuBPM, AmBPM |
| Functional tests | |
| Kidney biopsy |
AmBPM: ambulatory blood pressure measurement; AuBPM: automated blood pressure measurement; BSA: body surface area; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; T2D: type 2 diabetes; HTN: hypertension.
Initial patient assessment will often be continued with middle- to long-term follow-up, due to CKD or persistent risk factors for developing CKD. In other cases, the patient will be discharged following resolution of the acute process, if it bears no relationship to cancer or cancer treatment.
Multidisciplinary approachA multidisciplinary approach makes it possible to offer patients the best clinical outcomes with a positive impact on survival and quality of life.37 Multidisciplinary care requires a proactive, bidirectional relationship between different specialists.9,38
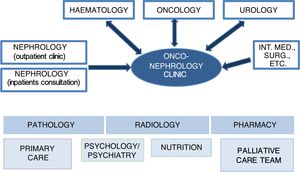
In onconephrology, nephrologists, oncologists, haematologists and urologists will comprise the core of the collaborative team. Participation by specialists in other areas, such as primary care, palliative care, psychology/psychiatry and nutrition, will also be essential in the care and follow-up of patients depending on their needs. Finally, specialists in pathology, radiology and pharmacy also play a significant role in the approach to diagnosis and treatment (Fig. 1).
Relationship with oncologyCommunication with oncologists must be ongoing and of a close nature with the objective of facilitating decision-making and patient follow-up.
Clinical sessions with multidisciplinary participation are very useful for assessing the risk/benefit ratio of potentially nephrotoxic treatments in patients at high risk of developing nephrotoxicity and patients with prior kidney disease. In this regard, strategies must be established for preventing or minimising nephrotoxicity associated with drugs and other agents (e.g. repeated use without suitable timing of intravenous radiological contrasts).
Relationship to urologyCollaboration between nephrologists and urologists is essential for ensuring proper management of patients with kidney cancer who present both acute kidney failure and CKD. Nephrologists should be familiar with the biology of kidney cancer, its treatment and its complications, and should be involved in the longitudinal care of these patients.39,40 They should actively participate in pre-operative assessment when deciding on partial or radical nephrectomy. If there has been prior CKD or there are risk factors for developing it must be taken into account. The objective in these patients is to preserve as much kidney parenchyma as possible, without giving up on the possibility of a cure with tumour resection, whenever possible. Patients with metastatic disease, exposed to systemic treatments with potential kidney toxicity (anti-vascular endothelial growth factor [anti-VEGF] therapy, mTor inhibitors or checkpoint inhibitors), also benefit from this collaboration between the two specialisations.
Furthermore, it is not uncommon for patients with other urinary tract tumours to develop acute or chronic kidney disease as a result of urology procedures that they undergo (urinary shunts, cystectomies, etc.).41 Hence, such patients are also candidates for a joint urology and nephrology approach.
Relationship to haematologyCharacteristically, onco-haematology constitutes an area of special relevance, as in many cases kidney impairment may facilitate the diagnosis of haematological disease and therefore the indication for its treatment. The course of kidney disease is used as an indicator of response to treatment and a marker of disease relapse.
In recent years, the term monoclonal gammopathy of renal significance has taken on a great deal of importance. This term is applied to cases that do not meet criteria for multiple myeloma and refers to a haematological disease consistent with monoclonal gammopathy of undetermined significance but associated with significant morbidity due to the severity of the kidney injury caused by the paraprotein. In these cases, close collaboration between nephrology and haematology is crucial, since the patient's kidney prognosis and vital prognosis will depend on early diagnosis and initiation of suitable chemotherapy (although there are no haematological criteria for treatment), aimed at eliminating pathogenic paraprotein production.36
Relationship to the kidney transplant teamAnother scenario in which multidisciplinary assessment proves key is kidney transplantation. The 2016 PROMETEO project, sponsored by the Sociedad Española de Trasplante [Spanish Transplant Society], demonstrated the importance of this fact.42 Involving oncologists in the process of adding a patient with a history of cancer to the transplant waiting list enables them to contribute precise information on the risk of cancer relapse. This makes it possible to determine the right time to add the patient and to establish the most beneficial immunosuppression plan for them. Such collaboration will also be necessary in the management of kidney transplant patients who develop cancer at some point in the post-transplantation period.43 A detailed assessment of treatment options should be conducted, taking into account the overall survival of the patient and the graft, and their quality of life.
Cancer and renal replacement therapyA multidisciplinary approach with participation by nephrologists is also essential in decision-making around renal replacement therapy. It takes on particular importance both when starting dialysis in a patient with active neoplastic disease and when suspending it. The decision-making process in onconephrology should be personalised and should incorporate the courses of both diseases — cancer and kidney disease. In this regard, the shared decision-making model may be useful.44 This model assesses clinical considerations and the general prognosis and integrates them with the patient's personal context in order to arrive at an informed decision. This requires a suitable assessment of the prognosis using scales of functional status, nutritional level, comorbidity and frailty. It also requires empathic and effective communication of that prognosis. This will allow for an approach to the different treatment options, with their respective pros and cons, with a careful examination of the patient's personal goals. Thus an informed treatment plan aligned with the patient's personal preferences may be formulated.
Onconephrology in SpainThe establishment of an onconephrology working group (ONCONEFRO) in 2018 within the Sociedad Española de Nefrología45 reflected recognition of the growing importance of this subspeciality in Spain and a commitment to implementing projects and studies in this area.
Between December 2018 and January 2019, the ONCONEFRO group conducted a survey to determine the current situation of onconephrology in Spain.
A total of 168 responses were received and nine hospitals that already had a dedicated practice as of January 2019 were identified: Hospital Clínic Universitari [University Clinical Hospital] (Valencia), Hospital Universitario Virgen del Rocío [Virgen del Rocío University Hospital] (Seville), Hospital Universitario Virgen Macarena [Virgen Macarena University Hospital] (Seville), Hospital Universitario Doce de Octubre [Doce de Octubre University Hospital] (Madrid), Hospital Clínic [Clinical Hospital] (Barcelona), Hospital Universitario Nuestra Señora de Candelaria [Nuestra Señora de Candelaria University Hospital] (Tenerife), Hospital Sant Joan de Déu [Sant Joan de Déu Hospital] (Barcelona), Clínica Universitaria [University Clinic] (Navarra) and Hospital General [General Hospital] (Alicante). The first two hospitals were the ones that had had a dedicated onconephrology practice for the longest period of time. Another nine hospitals also indicated that they planned to establish such a practice soon.
Despite the fact that 80% of those surveyed felt that onconephrology is an emerging subspecialisation that requires active participation by nephrologists, just 42% thought that it was possible to bring the project of open a monographic consultation for reasons of logistics and staffing.
Although such outpatient onconephrology clinics are known to have been established in other countries, preliminary data on outcomes are not yet available. In the United States, hospitals such as the MD Anderson Cancer Center in Houston, the Memorial Sloan Kettering Cancer Center in New York and the Dana-Farber Cancer Institute in Boston are increasingly adopting a more multidisciplinary model for cancer care.46
Cumulative experience with onconephrology outpatient clinic at two Spanish hospitalsIn Spain, we decided to analyse the initial data for a series of 199 patients from two hospitals with a monographic onconephrology consultations, assessed from the time of their establishment (Hospital Universitario Virgen Macarena in Seville, 161 patients, opened in October 2017, and Hospital Universitario Doce de Octubre in Madrid, 38 patients, opened in June 2018) to October 2019, with similar inclusion criteria at the two hospitals.
Tables 5 and 6 describe our series of patients in terms of baseline characteristics and kidney impairment characteristics.
Main characteristics of patients referred to an onconephrology practice.
| Total patients (N = 199) | |
|---|---|
| Age (years), Md (IR) | 68 (59−76) |
| Male sex (n) (%) | 129 (65) |
| Prior CKD (n) (%) | 131 (66) |
| Solid tumour/haematological disease (n) (%) | 112 (56)/87 (44) |
| Metastatic disease (n) (%) | 60 (35) |
| Time from oncology diagnosis to referral to the practice (months), Md (IR) | 20 (8−50) |
| Treatment line (n) (%) | |
| First line | 69 (70) |
| Second line | 18 (18) |
| Third or further line | 11 (12) |
| Cancer treatment at time of referral (n) (%) | |
| Chemotherapy | 48 (48) |
| Hormone therapy | 8 (8) |
| Immunomodulatory therapya | 7 (7) |
| Targeted therapiesb | 26 (26) |
| Checkpoint inhibitorsc | 11 (11) |
| CAR T cells | 1 (1) |
CAR T cells: chimeric antigen receptor T cells; CKD: chronic kidney disease; IR: interquartile range; Md: median.
Characteristics of kidney impairment.
| Total patients (N = 199) | |
|---|---|
| Serum creatinine (mg/dl), Md (IR) | 1.6 (1.3−1.9) |
| Serum creatinine in patients ppp referred for AKI (mg/dl), Md (IR) | 1.7 (1.4−2.1) |
| Glomerular filtration rate (ml/min/1.73 m2)a, Md (IR) | 40 (30−53) |
| Proteinuria (g/day), Md (IR) | 0.1 (0.1−0.6) |
| Haematuria (n) (%) | 39 (21) |
| [0,1–2]Electrolyte disorders (n) (%) | 39 (21) |
| Hyponatraemia | 10 (26) |
| Metabolic acidosis | 11 (28) |
| Hypomagnesaemia | 3 (8) |
| Hyperkalaemia | 5 (13) |
| Hypercalcemia | 9 (23) |
| Hypocalcemia | 1 (3) |
| Hypertension (n) (%) | 129 (65) |
AKI: acute kidney injury; IR: interquartile range; Md: median.
Some differences between the population with solid tumours (n = 112) and the population with haematological disease (n = 87) were noted. For example, with regard to reason for referral, CKD was the main reason in the haematologic group (67%), followed by AKI (24%). In the case of the population with solid tumours, the percentages were similar (42% CKD versus 43% AKI).
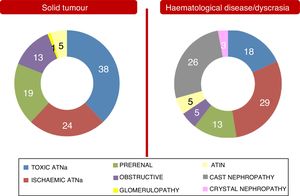
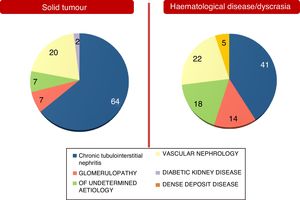
These differences between the two population groups proved more significant when an aetiology of AKI and an aetiology of CKD were analysed separately. They are shown in Figs. 2 and 3. Notably, CKD of undetermined aetiology increased in the case of the population with haematological disease (18% of cases), probably due to the impossibility of performing kidney biopsy (comorbidities, high risk, advanced age in many patients with monoclonal gammopathy of renal significance, kidneys found to be unfit for biopsy on ultrasound, etc.).
Regarding treatment, it should be noted that almost half the patients (49%) were on active cancer treatment when they were referred to the onconephrology practice. Of these, 38 required temporary suspension of this treatment due it its association with kidney impairment, 10 required permanent suspension without restarting any other treatment and 13 were switched to another line of treatment. Partial or complete improvement in kidney impairment in patients who underwent some sort of nephrologist-performed procedure occurred in 86 patients (86%). This reflects the importance of nephrology assessment in these patients.
Of the 199 patients, 25 underwent kidney biopsy (13%). The main pathology diagnoses were: monoclonal gammopathy of renal significance in seven patients (28%), paraneoplastic glomerular disease in six patients (24%), acute interstitial nephritis in four patients (16%) and tubulopathy due to cisplatin in one patient. In two cases, the diagnosis remained unclear even with biopsy, and in the five remaining patients (20%), the histology diagnosis was not related to the neoplastic disease/blood dyscrasia or the treatment thereof.
Overall, from a clinical point of view, a possible relationship between kidney disease and cancer was established in the different populations with solid tumours or haematological disease (Table 7). It was believed that there was no relationship between kidney disease and cancer in 38% of patients with solid tumours and 36% of patients with haematological disease. Kidney impairment was related more to haematological disease, strictly speaking, in 37% of cases and to cancer treatment (whether medical or surgical) in 40% of the population with solid tumours. There was a higher percentage of an unclear relationship in those with haematological dyscrasia/tumour (19%).
Finally, around 20% of patients referred to the practice died during the follow-up period. The leading cause of death was the cancer itself in 80% of cases. Infections, cardiovascular disease and terminal end stage chronic kidney disease corresponded to 6%, 6% and 3% of all other causes, respectively.
Based on our experience, there are no initial difficulties in establishing this type of practice. It is important to present the project to the other specialisations involved, in order to stress the value of collaborative work and introduce the referring "onco-nephrologist".
In general, hospitals' own experience with the practice has been satisfactory, both for the other specialisations involved and for the patients themselves.
Requirements and recommendations for opening a dedicated onconephrology practiceThanks to the ONCONEFRO survey and the experience of hospitals with an established practice, some requirements have been identified and are set out in Table 8. A sufficient population area is necessary to establish the practice, of between 250,000 and 500,000 inhabitants, consistent with other previous publications.38 This would allow an annual flow of approximately 150 patients.
Recommended requirements for opening a dedicated onconephrology practice.
| Reference hospital population area: 250,000−500,000 inhabitants |
| Oncology and haematology units present in the hospital, with training of multidisciplinary working groups |
| Common electronic system access and shared patient data |
| Recommended annual patient flow (at least 150 patients) |
| Recommended time available for check-ups: 15−20 min |
| Possibility of coordinating patients' different hospital visits |
| Possibility of early response to preferential/emergency interconsultations |
As the practice's workload increases, it may be necessary to split the workload into patients with solid tumours and patients with haematological dyscrasia so as to facilitate the day-to-day work.
Hospitals that already have a practice have also recommended direct contact with haematology, oncology and urology departments. The establishment of multidisciplinary working groups for approaching specific cases is essential for streamlining management and decision-making. As these patients may develop an acute disorder at any time, it is recommended that the work be organised in such a way as to ensure the possibility of emergency care in these cases.
It is desirable for there to be at least two people responsible for the onconephrology programme in each unit. This would enable distribution of clinical care and duties. Onco-nephrologists must constantly update their training. They must also be familiar with the oncology terminology used. This may prove difficult in day-to-day work, due to the high existing healthcare burden on nephrology units. Direct contact with oncologists is essential for determining the prognosis of the patient's cancer. This too may prove an arduous task, given the subspecialisation by body system within oncology itself.
When establishing the practice, it would be advisable to start a suitable registry of information on a local level. Unification of data collection on a national level would enable incidence and prevalence studies of these complications, clarification of kidney injury associated with cancer treatment, determination of its course and management, and identification of areas for improvement in the care of these patients.
Multidisciplinary training meetings wherein pathways and protocols may be established are vitally important. It is desirable to have "nephrological" training aimed at the other specialisations involved. This would allow for the use of a common language and early identification of kidney disease. Assessment of the outcomes achieved should also constitute a priority objective.
Final considerationsThe establishment of dedicated onconephrology practices results in better care of patients with cancer who develop an acute or chronic kidney abnormality or electrolyte disorder. However, it is subject to some requirements and recommendations. Individualised adaptation at each hospital will be essential for the success and continuity of the practice.
A multidisciplinary approach among the different specialisaties involved must be taken with the objective of optimising decision-making and engaging in suitable patient management overall. The patient's prognosis, quality of life, personal preferences and values must be borne in mind throughout the process, especially with regard to decisions around renal replacement therapy. Within nephrology itself, a joint approach with the kidney transplant and dialysis team is often needed.
The series of practices we analysed showed overall a high percentage of patients with CKD and AKI as reasons for consultation.
We observed a low number of kidney biopsies performed. As a possible explanation for this, in some cases kidney biopsy was deemed unnecessary due to the absence of a clinically justified connection between the patient's cancer and kidney disease. In a non-negligible percentage of patients, kidney biopsy was not performed as it was technically impossible (comorbidities or unfit kidneys).
Manifest differences were seen in aetiologies of AKI and CKD between the two patient groups. In the case of the group with solid tumours, there was a stronger relationship between kidney disease and cancer treatments used (medical or surgical). In the case of the group with dyscrasia, kidney impairment was related more to haematologic disease, strictly speaking.
Worthy of note was the confirmation that a high percentage of patients with kidney disease associated with neoplasia or blood dyscrasia benefited from assessment at the practice as it enabled optimisation of cancer treatment and amelioration of kidney impairment.
The establishment of unified, registered databases may be useful for analysing clinical data in daily practice and for conducting multicentre prospective studies. Onconephrology practices should be a starting point for the achievement of this objective on a national level and for the growing development of this emerging subspecialisation.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank all the coordinators of the Spanish onconephrology group for their support in the conduct of this study (Patricia García, Isidro Torregrosa, Luis F. Quintana and Eduardo Gutiérrez). We would also like to thank Miguel Ángel Solís and Carmen Ramos from Hospital Clínic Universitari in Valencia, Virginia Cabello from Hospital Universitario Virgen del Rocío in Seville, Nuria García from Clínica Universitaria in Navarra, Pedro Arango from Hospital Sant Joan de Déu and Miguel Perdiguero from Hospital General in Alicante for their help with the survey conducted on onconephrology practices.
Please cite this article as: Alonso F, Auñón P, Cavero T, Salgueira M, Praga M, Quiroga B, et al. Consulta monográfica de onconefrología. Justificación y puesta en marcha. Nefrologia. 2021;41:154–164.