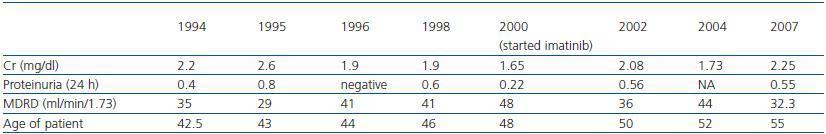La leucemia mieloide crónica (LMC) es una enfermedad mieloproliferativa caracterizada por la expansión clonal de células mieloides que expresan la proteína de fusión BCR-ABL, responsable de los efectos oncogénicos de la LMC. La terapia actual en el tratamiento de LMC es el inhibidor de la BCR-ABL tirosín-kinasa, imatinib. Aunque este fármaco ha demostrado mejorar la supervivencia en pacientes con LMC, su papel en el contexto del trasplante renal no ha sido ampliamente descrito en la literatura. Presentamos un caso de remisión molecular de LMC en un varón de 55 años con un segundo trasplante renal, hepatitis C y con riesgos cardiovasculares e inmunológicos asociados.
INTRODUCTION
Chronic myeloid leukaemia (CML) is a myeloproliferative disease characterised by clonal expansion of myeloid cells.1 The pathognomonic cytogenetic anomaly in CML is the Philadelphia chromosome,2 which is produced by reciprocal translocation of the two long arms of chromosomes 9 and 22.3 This translocation generates the BC-ABL fusion protein, which is responsible for the oncogenic effects in CML.
On only two occasions does the literature describe the use of Imatinib (Gleevec, Novartis), a BCR-ABL tyrosine kinase inhibitor for CML treatment, in a kidney transplant patient;4,5 there are no described cases in a patient with a second transplant with hepatitis C and high cardiovascular and immunological risk.
CASE REPORT
Male patient 55 years of age with CML that reached the chronic stage following his second kidney transplant. The patient started haemodialysis for mesangiocapillary glomerulonephritis in 1976 when he was 24, and received a kidney transplant from a live donor in 1983. His immunosupressive treatment was azathioprine and steroids. In 1989, six years after the first transplant, he suffered an acute myocardial infarction. Two years later, he began haemodialysis treatment once again due to chronic rejection of the allograft.
In 1994, when the patient was 42, he received a second kidney transplant from a 44 year old female donor. The donor and the recipient share three HLA alleles (HLA A3, B8 and DR3). The panel reactive antibody (PRA) score was 29%, probably due to various blood transfusions during the course of the first transplant, and to the transplanted organ itself. Immunosuppressants initially administered following the second transplant consisted of steroids (0.5mg/kg/day), azathioprine (2mg/kg/day) and cyclosporine A (CyA) (10mg/day).
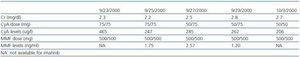
Following the transplant, the patient developed postischaemic acute tubular necrosis, which required two subsequent surgical procedures one month after the transplant to correct a urinary fistula. At the time of the surgical procedure, a kidney biopsy was performed which revealed acute stage 1 rejection; steroids were administered as rescue treatment (1g in four doses). Two years later, the azathioprine was substituted with mycophenolate (tables 1 and 2) because of decreased renal function with CyA.
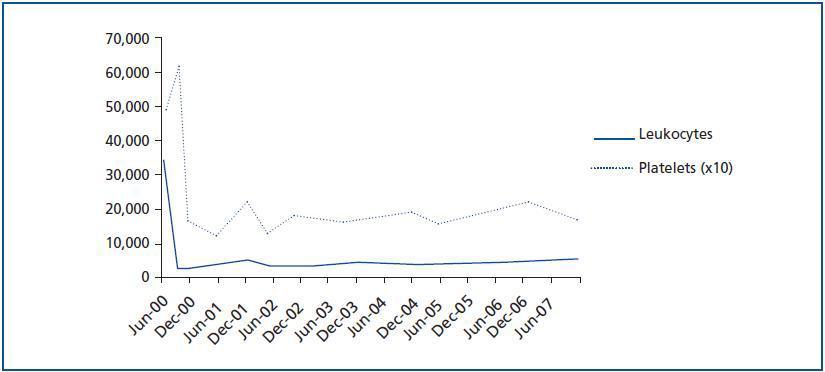
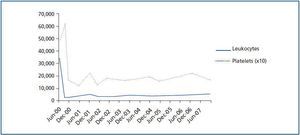
The patient¿s progress was satisfactory until June 2000, when a routine analysis revealed significant leukocytosis (34.5 x 109/l) and thrombocytosis (487 x 109/l) (figure 1). The patient experienced bilateral hip pain, but had no other symptoms. The examination was normal with no signs of spleen or liver enlargement.
An MRI scan of the hip showed diffused bone marrow involvement in almost all bones. The bone marrow aspiration and biopsy confirmed granulocytic and megakaryocytic hyperplasia compatible with CML. No fibrosis was observed in the bone marrow and the patient was low-risk according to the Sokal index.
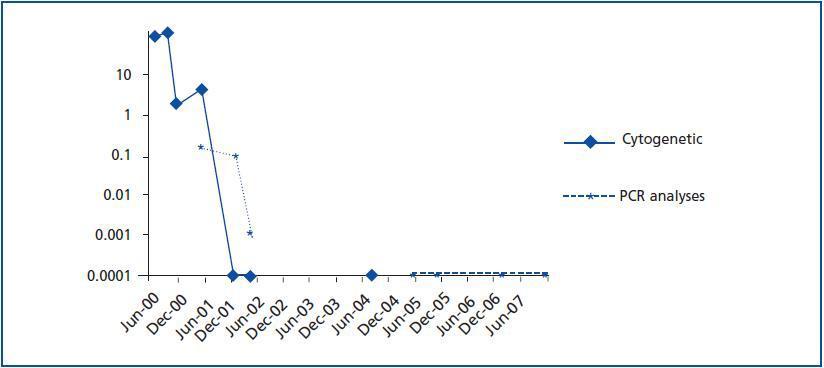
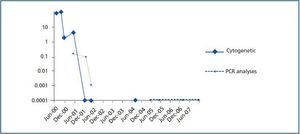
The presence of the Philadelphia chromosome (46XY t[9;22][q34;q11]) was detected by analysing the bone marrow karyotype. Subsequent cytogenic molecular analyses with fluorescent in situ hybridation (FISH) revealed the presence of the BCR-ABL fusion gene in 90% of the nuclei. The RT-PCR confirmed the typical rearrangement of p210 BCR-ABL with the fusion transcript b2a2. During two months, the patient was treated with hydroxyurea, with no results. In September of this year, the patient was placed in a clinical trial of Novartis STI571-0113 and received 400mg of Imatinib daily; he showed a good tolerance of the drug with no adverse effects. The patient went into complete, sustained haematological remission, complete cytogenetic remission, and molecular remission at two, six and nine months respectively, and this continues to be the case today (figure 2). Fourteen years after his second transplant, the patient maintains an acceptable level of renal function (stage III chronic renal disease) and has not experienced acute rejection, decreased hepatic function or new cardiovascular events (table 1).
DISCUSSION
This case shows that Imatinib is a safe, effective drug for the treatment of CML in kidney transplant patients. Our patient responded rapidly to the treatment: peripheral blood and bone marrow became normal in the first two months, and cytogenetic and molecular remission occurred during the first year of treatment. These results are in line with IRIS1 study, which showed a complete cytogenetic response in 89% of patients with a low Sokal index, and a survival rate of 83%. Lastly, that study and our patient¿s case both show that an early cytogenetic and molecular response is associated with better long-term evolution and a lower risk of progression to the accelerated phase or blast crisis.6
The use of Imatinib in this patient may also have had a beneficial immunosuppressant effect for the transplanted kidney, as suggested by recent in-vitro and animal studies showing that this drug may have immunomodulating effects. Appel et al. showed that Imatinib can inhibit the differentiation of CD34+ stem cells into dendritic cells (DC). Furthermore, T-cell activation may be inhibited, since DC cells¿ secretion of cytokines and chemokines decreases.7 Imatinib also inhibits the development of monocytes and macrophages in bone marrow progenitor cells.8 Lastly, Dietz et al. showed that Imatinib can inhibit T-cell proliferation by arresting the proliferation of lymphocytes in G0 phase.
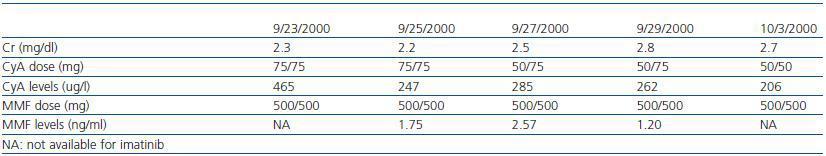
Some studies suggest that imatinib in the presence of cyclosporine can cause renal failure due to acute tubular necrosis.10,11 Despite receiving both drugs, our patient¿s renal function remained stable from the moment when treatment with Imatinib was started. Creatinine levels oscillated between 1.75 and 2.4mg/dl with low levels of proteinuria (the maximum proteinuria level was 0.56g/dl), (table 1).
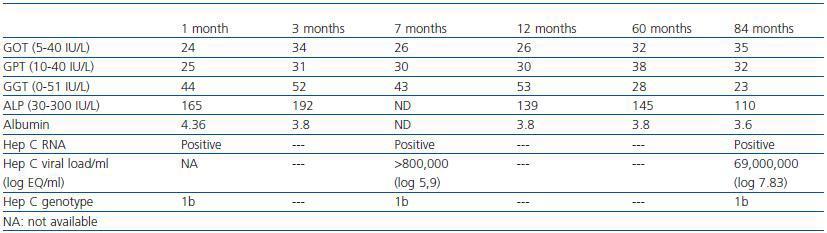
Serious hepatic side effects have also been described, including fulminant liver failure associated with imatinib treatment.12-15 Our patient, who was positive for HCV, experienced no changes in liver function during treatment. Liver function remained within the normal range at all times, despite having detectable HCV RNA in blood plasma, which tends to be the case for most HCV patients following a transplant.16 (Table 3).
Imatinib, according to the manufacturer, may have adverse effects in cardiac patients due to the risk of heart failure. Our patient, who suffered a myocardial infarction at the age of 37, has experienced no adverse effects and his cardiac stress test this year was normal.
We conclude that imatinib is a safe and effective treatment for kidney transplant patients with CML. Furthermore, in vitro and animal studies suggest that the drug may have immunomodulating effects that could be beneficial for a transplant.
Figure 1.
Table 1. Patient's renal function over the last 13 years
Table 2. Creatinine, cyclosporin (CyA) and mycophenolate (MMF) levels during treatment with imatinib
Table 3. Liver function and hep C status from one month after starting treatment
Figure 2.