To the Editor,
Methylmalonic acidemia with homocystinuria (MMAH) is a rare congenital metabolic and heterogeneous disorder affecting vitamin B12 or cobalamin (cbl) metabolism. The disorder causes a reduction in the levels of adenosyl and methylcobalamin coenzymes, in turn reducing the activity of their respective enzymes, methylmalonyl-CoA mutase and methionine synthase. This results in the accumulation of methylmalonic acid and homocysteine in the blood and tissues, with an increase in the urinary excretion of both compounds1. Various forms of the disease have been described: cblC, cblD and cblF. Neonatal presentation of this condition includes failure to thrive, encephalopathy, psychomotor retardation, haematological abnormalities of the three series and renal damage1. We present two cases diagnosed in our department, who died from atypical haemolytic uraemic syndrome (HUS) associated with severe kidney failure.
The first case was a 25-day-old male, admitted due to bilious vomiting and liquid bowel movements which had started four days earlier. He was the second son of first-cousin parents. On admission he presented mild malnutrition, hypotonia and hypoactivity. He had normochloraemic metabolic acidosis. Following slight improvement on being subjected to complete fasting, feeding was started; poor tolerance, neurological deterioration, pancytopenia and liver and renal failure were observed.
Subsequently, on initiating parenteral nutrition, microangiopathic anaemia was reported together with increased thrombocytopenia (haemoglobin 6.7 g/l, platelets 10,000/mm3) and worsening of renal failure. Atypical HUS was diagnosed. In addition, he experienced various convulsive episodes, with encephalopathic findings in the electro-encephalogram. He died 20 days after admission with severe kidney failure (creatinine 1.3mg/dl, urea 193mg/dl, potassium 6.6mEq/l).
The second case was a 24-day-old male, who was taken to hospital due to 7% weight loss following birth, hypotonia and general malaise. The parents were also first cousins. He was admitted with a diagnosis of suspected sepsis (increase of acute-phase reactants and positive haemoculture for coagulase-positive staphylococcus). He also had normochloraemic metabolic acidosis. Antibiotics were prescribed and the patient continued with complete fasting, with good clinical response. Poor tolerance, respiratory difficulty, neurological deterioration, pancytopenia and liver failure were observed on beginning nutrition. At that time, he was diagnosed with dilated myocardiopathy with reduced ejection fraction, which normalised after suspending nutrition. Parenteral nutrition was subsequently started, when kidney failure occurred (oligoanuria, creatinine 1mg/dl, urea 90mg/dl), accompanied by anaemia and thrombopenia (haemoglobin 7.7g/l, platelets 21,000/mm3). Therefore, continuous venovenous haemofiltration was started. Although the presence of schistocytes was unknown, we suspected that he suffered from atypical HUS. Cerebral echography showed severe cortical atrophy. 30 days after admission the diagnosis of methylmalonic acidemia with homocystinuria was confirmed. Given the unfavourable prognosis, we decided upon a limitation of therapeutic effort.
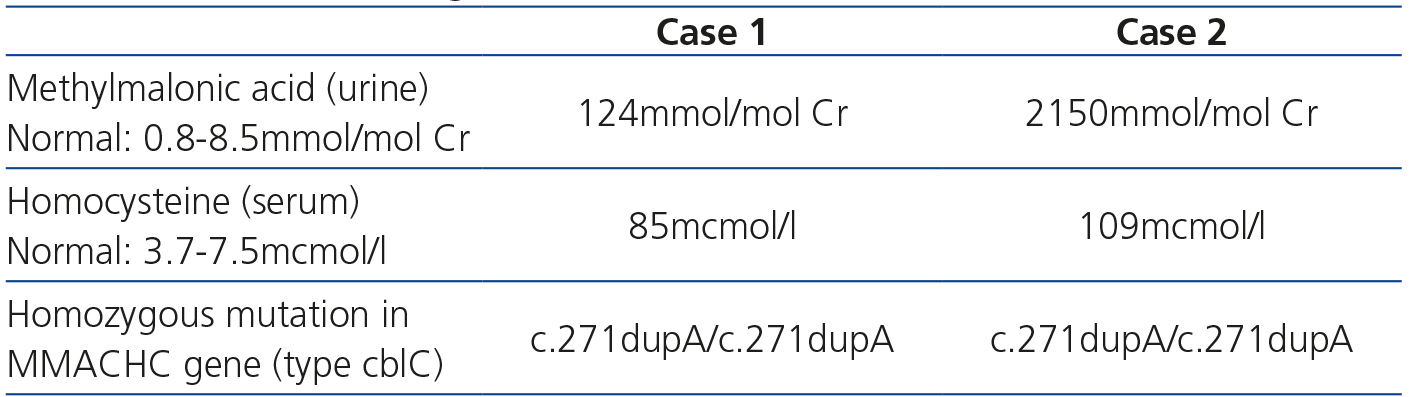
The most noteworthy data from the metabolic and genetic study of both patients, required for diagnosis, are shown in Table 1. Our patients were suffering from the most common variant of the disease (cblC), which is caused by homozygous or compound heterozygous mutations in the MMACHC gene [methylmalonic aciduria (cobalamin deficiency) cblC type, with homocystinuria], which is located on the 1p34 chromosome.
A symptom-free period is typical in methylmalonic acidemia with homocystinuria, since for clinical symptoms to appear, protein intake is required, with the consequential accumulation of methylmalonic acid and homocysteine. This explains why, in our patients, deterioration was observed on restarting feeding, whether enteral or parenteral. At times, there was a larvate clinical sign which was precipitated by intercurrent disease, often an infection, as occurred in case 2. Dilated myocardiopathy (case 2) is also described as a complication, of which a case diagnosed prenatally was reported2, as well as other cardiac disturbances in relation to thromboembolisms.
The pathogenesis of thrombotic microangiopathy is related to the increase of plasma methylmalonic acid and homocysteine levels. The latter modifies the vascular endothelial’s antithrombatic properties by interfering in the inhibition of platelet aggregation mediated by nitric oxide, which favours the union of the tissue plasminogen activator with the endothelial. This results in an increase of the endothelial expression of procoagulants. In addition, homocysteine thiolactone, homocysteine metabolite, can cause cell damage by inducing intracellular accumulation of free radicals and methylmalonic acid can interfere in the mitochondrial metabolism of renal cells. Association with HUS is uncommon, although described, above all, in newborns3,4, as was confirmed in case 1 and suspected in case 2. At birth, many patients already have kidney failure, which could be reversible with early treatment (hydroxocobalamin, trimethylglycine, folate and protein restriction), which did not occur in our cases given the late diagnosis4,5. Consequently, early clinical suspicion is fundamental for trying to improve renal function as much as possible.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Biochemical and genetic data