Methanol poisoning is a rare and serious emergency. It produces acidosis, visual and cognitive alterations.
There are not many cases described as those that we present here, associated with methanol inhalation.
Case 1A 21-year-old woman with no medical history and in no medications who came to the emergency room complaining of blurred vision. She presents progressive decrease in vision during the last 24h and respiratory distress. She refers a continued inhalation of universal solvent (methanol: 10–25% and toluene: 50–75%) during the last 5 days. She negates other toxics consumption.
She reports inhalation of solvent since she was 13 years old that was stopped 5 years ago.
Physical exam: BP 123/87mmHg; HR 88bpm; O2 saturation 100% and breathing at 40rpm; sleepy; reactive mydriatic pupils and normal oculomotor exploration. Finger-nose dysmetria (Table 1).
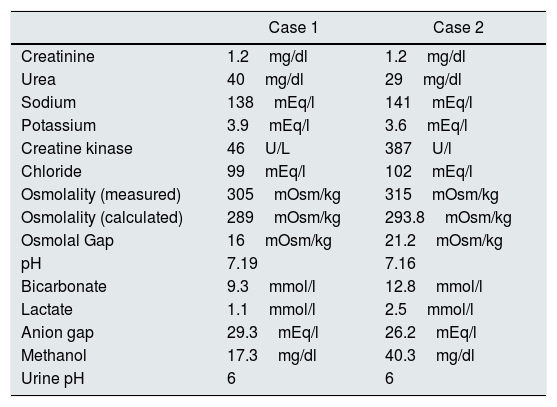
Complementary tests. Both patients show, metabolic acidosis with high anion gag (formic acid) and high osmolal gap (methanol).
| Case 1 | Case 2 | |
|---|---|---|
| Creatinine | 1.2mg/dl | 1.2mg/dl |
| Urea | 40mg/dl | 29mg/dl |
| Sodium | 138mEq/l | 141mEq/l |
| Potassium | 3.9mEq/l | 3.6mEq/l |
| Creatine kinase | 46U/L | 387U/l |
| Chloride | 99mEq/l | 102mEq/l |
| Osmolality (measured) | 305mOsm/kg | 315mOsm/kg |
| Osmolality (calculated) | 289mOsm/kg | 293.8mOsm/kg |
| Osmolal Gap | 16mOsm/kg | 21.2mOsm/kg |
| pH | 7.19 | 7.16 |
| Bicarbonate | 9.3mmol/l | 12.8mmol/l |
| Lactate | 1.1mmol/l | 2.5mmol/l |
| Anion gap | 29.3mEq/l | 26.2mEq/l |
| Methanol | 17.3mg/dl | 40.3mg/dl |
| Urine pH | 6 | 6 |
The decreased potassium values and the high urinary pH for the level of acidosis indicates the presence of toluene.
20-Year-old man, consumer of inhaled solvent since he was 8 years old. Intoxicated a year ago by glue (toluene) with optical atrophy and 10% visual acuity.
He came to the hospital complaining of general malaise, abdominal pain and vomiting during the last 24h. The patient refers a continued inhalation of solvent for 3 days.
Physical examination: BP 115/82mmHg; HR 110bpm and respiratory rate of 38rpm. Upon his arrival he was conscious and oriented, but with tendency to sleep in the following hours. Oculomotor exploration with mild reactive mydriasis.
Given the clinical suspicion of intoxication by methanol and toluene whose levels are not available immediately, it was decided to administer bicarbonate and ethanol and perform a high flow hemodialysis session for 8hours.
Case 1: At the end of the treatment, despite the correction of the electrolyte alterations, there was little improvement in the level of alertness and vision, with ophthalmological examination and MRI performed without finding. It was decided to repeat a new session of hemodialysis for 4h, with discrete cognitive and discrete visual acuity improvement without total recovering at discharge.
Case 2: During the hemodialysis session the alert level progressively improved. He did not need a second session.
DiscussionToluene is in glues and solvents. It is metabolized to hippuric acid that is rapidly secreted by the kidney producing acidosis with normal or minimally elevated AG and hypokalemia due to volume contraction with RAA stimulation. It is lipophilic, so it acts rapidly in the CNS (euphoria, hallucinations, ataxia, confusion-coma, optic neuropathy, …). The treatment is limited to correct hydroelectrolytic alterations.
Methanol is present in many industrial and domestic products. It is common to find cases of intoxication by ingestion and transdermal absorption, but there are few cases described of intoxication by inhalation.
Symptoms depend on the level of exposure: abdominal discomfort, CNS alterations, even coma and death, and visual disturbances (blurred vision, photophobia, scotomas, decreased acuity and even blindness). The presence of non-reactive mydriasis is a sign of poor prognosis and indicates the irreparable loss of vision.1–3
It is characterized by metabolic acidosis with high anion gap (accumulation of formic acid) and/or high osmolal gap (methanol accumulation).
The value of osmolal and anionic gap depends on the time elapsed after intoxication. Initially, it has a high osmolal gap, subsequently it decreases when methanol is metabolized to its acid metabolites, increasing the anionic gap and decreasing the osmolal gap.4 Therefore, in our cases that have been exposed to sustained slow poisoning, the anion gap is higher than osmolal.
The methanol level can be estimated by means of the osmolal gap: by subtracting the normal gap (10–12mOsm/kg) and multiplying by the molecular weight of methanol (32g/mol).1
Methanol is metabolized via alcohol dehydrogenase (ADH) giving rise to formic acid, responsible for acidosis and visual damage by inhibiting mitochondrial function in the retina. Treatment includes the administration of ethanol, with 10–20 times greater affinity for ADH, with a complete inhibition at a concentration of 100mg/dl.5,6
The levels of formic acid are directly related to morbidity and mortality.
In our cases, the high osmolal and anionic hiatus orientate to methanol as the main toxic, although hypokalemia and absence of acid urine indicate some effect of toluene.
General indications of hemodialysis include a high methanol level (>50mg/dL), metabolic acidosis, and visual or mental changes. It is well dialyzed because it is a small molecule (32Da), not bound to proteins with a distribution volume of 0.6–0.7l/kg.7–9
Methanol poisoning is a serious condition whose initial treatment includes performing hemodialysis, rapidly reducing the levels of alcohol and its metabolites, decreasing morbidity and mortality. Since immediate determination of methanol is hard to obtain, the treatment must be extended until the improvement of the cognitive level and correction of the electrolyte alterations that are related to the toxic levels.
In cases of inhalation poisoning, the values of anionic gap are more important than the osmolal gap because they are usually slow intoxications, better tolerated, which delay the arrival of the patient in the emergency room. The attitude towards these patients has to be dictated by the clinic findings rather than the analytical data, since it is a serious situation that can lead to the permanent reduction of visual acuity and even death.
Please cite this article as: Robledo C, Saracho R. Intoxicación por metanol por inhalación de disolvente. Nefrologia. 2018;38:679–680.