From 2000, Mesoamerican region has reached an important rate of chronic kidney disease of unknown etiology. Under the name of Meroamerican Nephropathy (MeN) several hypotheses (including dehydration, heat stress, environmental or toxic exposure or even infections) have tried to explain the etiology this new disease. MeN affects young men, agricultural workers exposed to high temperatures. MeN courses with unspecific symptoms as low-grade fever and dysuria and progressive kidney disease with impaired renal function and hydroelectrolyte disturbances. The diagnosis requires kidney biopsy showing tubule-interstitial nephritis (usually at chronic stage). Although MeN conditions a high morbi-mortality in endemic regions, there is a lack of specific treatment and only preventive measures have demonstrated some effect of prognosis (avoid heat stress, constant hydration). In this review we aim to summarize the available information of MeN, illustrating the information in a case report.
Desde el año 2000, la región de Mesoamérica ha presentado una elevada incidencia de casos de enfermedad renal crónica de origen desconocido. Bajo el nombre de nefropatía endémica mesoamericana (NeM) han concurrido numerosas hipótesis incluyendo la deshidratación, el estrés por calor, la exposición a contaminantes ambientales e incluso determinadas infecciones, sin que actualmente exista unanimidad en la etiología de dicha patología. La NeM afecta principalmente a varones jóvenes dedicados a actividades agrícolas con antecedente de exposición a temperaturas especialmente elevadas. Clínicamente cursa con síntomas inespecíficos como febrícula y disuria y analíticamente con deterioro de función renal y alteraciones hidroelectrolíticas. El diagnóstico exige de la realización de una biopsia renal que muestra invariablemente datos de nefritis tubulointersticial principalmente crónica. A pesar de que la NeM condiciona una elevada morbimortalidad en las regiones endémicas, no existe un tratamiento específico por lo que la prevención, basada en disminuir la exposición a elevadas temperaturas y asegurar el correcto estado de hidratación son de gran importancia. En la presente revisión, y basándonos en un caso clínico, actualizamos la evidencia disponible sobre un problema de salud pública con relevantes consecuencias renales.
Key concepts
- •
Endemic Mesoamerican nephropathy (MeN) is a chronic tubulointerstitial disease of tropical countries, affecting agricultural workers exposed to high temperatures.
- •
Although the etiology of MeN is not entirely clear, it can be assumed that the etiology is multifactorial that includes continuous episodes of dehydration, use of certain pesticides or exposure to metals and a certain genetic predisposition.
- •
Renal histological involvement includes chronic tubulointerstitial damage, which is constant, and glomerular involvement in final stages. In the initial stages, there is evidence of acute tubulointerstitial involvement.
- •
Despite its high morbidity and mortality, there is no effective treatment, so prevention based on hydration and avoiding certain toxic agents is a fundamental step to avoid the development of chronic kidney damage.
Let’s use a clinical case to guide this Review. This is a 46-year-old man, native of El Salvador, and with no relevant past medical history who was admitted for acute renal failure AKIN-3.
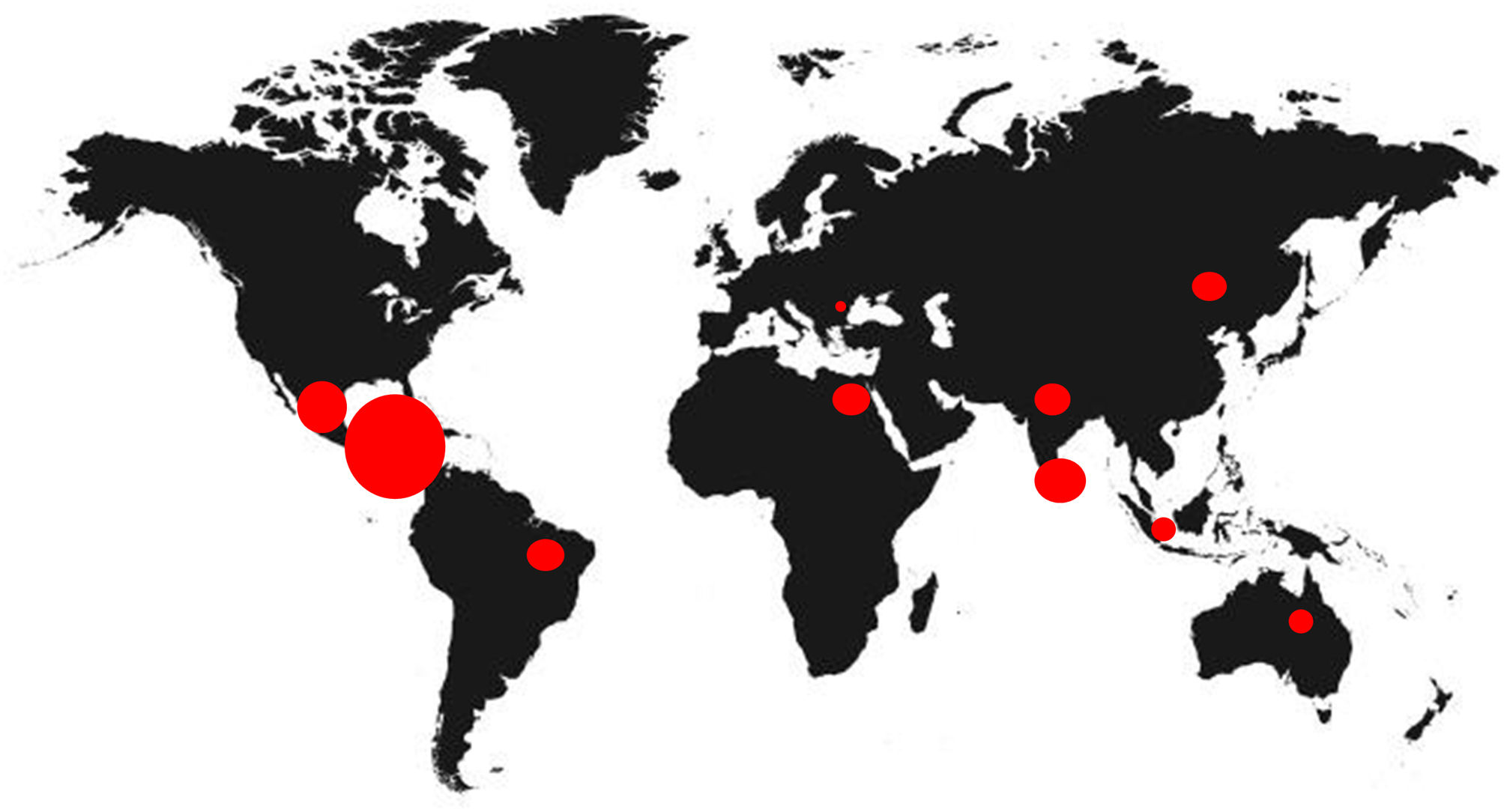
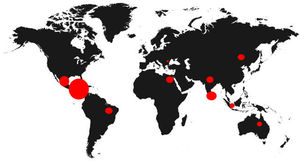
Since 2000, the Mesoamerican region including Southeast Mexico, Guatemala, El Salvador, western Nicaragua and northwestern Costa Rica, has witnessed an epidemic of cases of chronic kidney disease (CKD) of unknown etiology under the term endemic Mesoamerican nephropathy (MeN).1 The first reference of this new disease was an epidemiological description in a study published by García-Trabarino et al. after the evidence of a significant upturn of patients with advanced CKD in El Salvador.2 In this publication, 205 incident dialysis patients were analyzed, observing that at least 67% had no known risk factors for the development of kidney disease, but they did have a high exposure to pesticides and insecticides.2 Since then, the incidence of CKD of unknown etiology has increased considerably in Central America, but also in other tropical regions of the world, such as Sri Lanka or India, acquiring the broader name of chronic interstitial nephritis of agricultural communities (CINAC) (Fig. 1).3,4
In addition, the NeN is now the leading cause of premature death in young adults in the endemic regions.5 As compared with neighboring countries, the Bajo Lempa region, in El Salvador, has an incidence of advanced CKD on renal replacement therapy (RRT) that is 7 fold increased, and CKD mortality is also increased 30 times.6
Despite the alarming data derived from MeN, there is little evidence on the mechanisms involved in its development, the criteria for diagnostic, and the possible treatments. The objective of this manuscript is to perform a review on MeN taking as a common thread an illustrative clinical case.
Etiology and risk factorsThe patient refers that for 14 years he worked in the farm, in cotton fields, together with his family (father and sister), and both also had chronic kidney disease of unknown origin.
MeN typically affects young men from agricultural communities located in the mentioned geographic areas, characterized by a warm climate, with exposure to high temperatures, environmental pollutants, and pesticides have been postulated as fundamental triggers in the pathogenesis of this nephropathy.
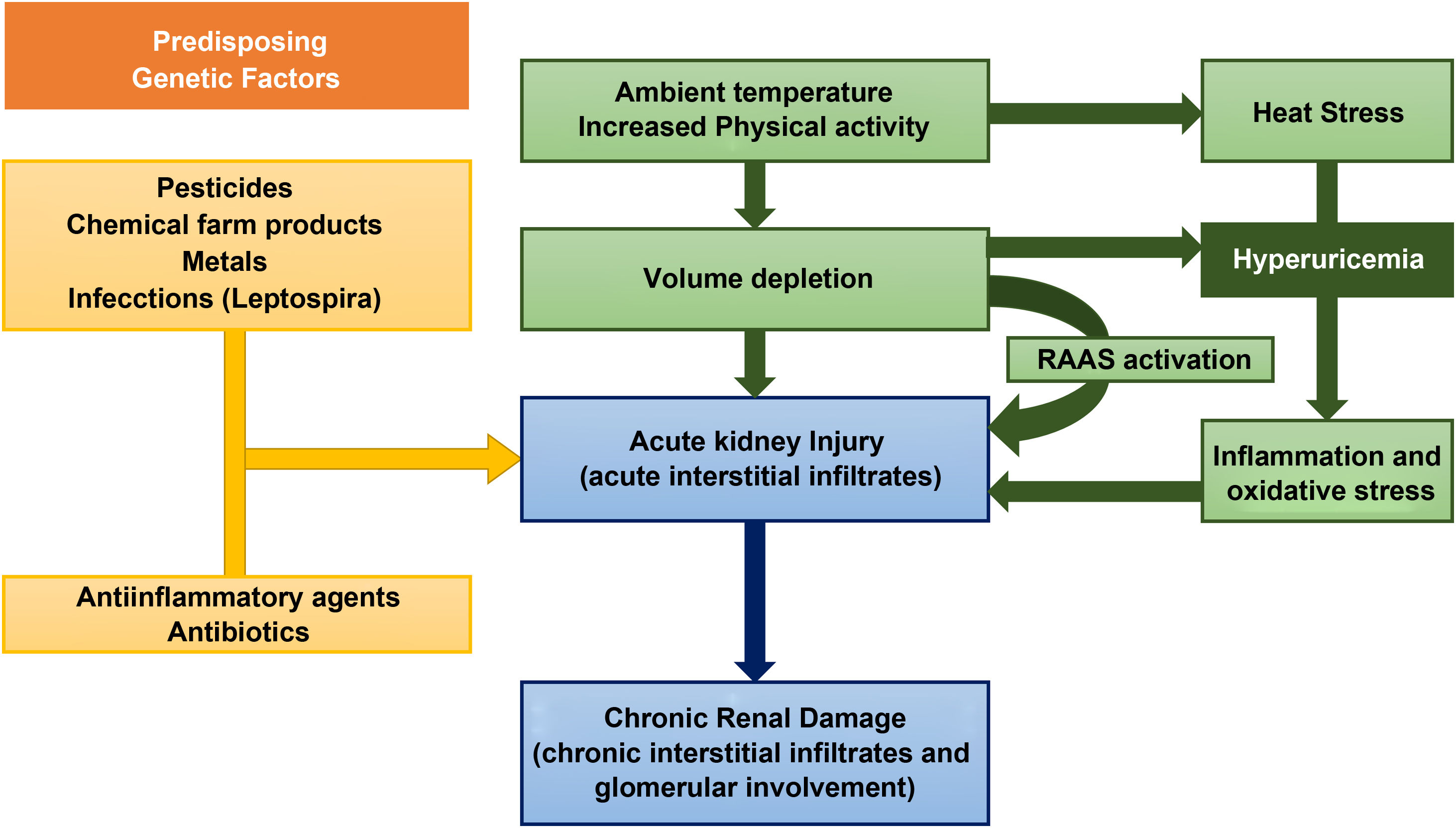
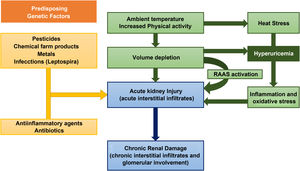
However, the current evidence on the causes of this disease is very limited. The risk factors for the development of MeN include, environmental, occupational and hereditary factors (Fig. 2).7
As for environmental factors, dehydration from any cause is considered by many authors as the main pathogenetic mechanism.8 Intensive physical activity, heat stress, and the high temperatures that workers are exposed lead to recurrent episodes of dehydration (and, consequently, repeated episodes of acute renal failure) in which the loss of water is greater than sodium, producing an increase in plasma osmolarity.9 This hyperosmolarity activates two main mechanisms: the release of vasopressin in the posterior pituitary and the enzyme aldose reductase.10 The latter converts glucose to sorbitol, which can lead to the formation of fructose, a substrate for fructokinase in the proximal tubule. This reaction, which increases the concentration of uric acid inside the cells of the proximal tubule, is capable of stimulating the inflammatory mediators responsible for tubulointerstitial injury and the progressive decrease in glomerular filtration.11,12 In addition to the loss of salt and water, the muscle damage that occurs under the previously mentioned conditions causes the release of nucleotide, leading to hyperuricemia and uricosuria. The concentration of uric acid in urine may exceed the solubility in urine leading to the formation of crystals of urate. These are mechanisms that contribute to the tubular damage of MeN.13 However, this mechanisms are not enough to explain the pathogenesis of MeN, that is almost exclusive of endemic areas with an increased incidence despite the introduction of mechanized farming machinery or the extrarenal involvement of the disease.14
Chemicals used in agriculture, such as glyphosphate and paraquat, and heavy metals present in the environment such as lead, arsenic, cadmium and silica have been the subject of discussion as factors involved in the pathophysiology of MeN, although without sufficient evidence to support conclusions about its pathophysiological implication. In fact, data derived from some published meta-analyzes agree on the lack of a sufficiently conclusive association between chemicals and pesticides with the development of MeN.15,16
However, some studies have shown an association between certain metals, such as silica and the development of CKD.17 Silica is one of the metals found in the ashes produced by burning of sugar cane, a very common occupational activity among patients with MeN.8
Other hypotheses, which needs to be confirmed, is the simultaneous development of MeN and certain infections, more specifically zoonoses. In a recent review, Yang et al. hypothesized the epidemiological association between leptospira infections and CKD of unknown cause, which MeN coincide geographically with the areas in which NeM had been described. In fact, the search for an environmental or occupational factor that could relate the MeN of sugarcane workers and the nephropathy from Sri Lanka, with very similar tubulointerstitial damage, showed anti-leptospira serology as a possible causal agent.18,19
Finally, genetic susceptibility has been shown in some families with CKD of unknown origin. Some studies have found an increase in urine biomarkers of kidney injury such as lipocalin associated with neutrophil gelatinase (NGAL) and N-acetyl-d-glucosaminidase (NAG) in children and adolescents with MeN with no history of working in the farm; this verifies an association between values of urine biomarkers of kidney injury and the development of MeN.20,21 All this could point to a genetic predisposition or even the association of accumulated doses of certain substances from an early age with subclinical kidney damage.8
The etiology of MeN has not been defined yet but it is probably due to the sum of certain predisposing factors such as dehydration, exposure to certain metals or the genetic characteristics that together trigger the characteristic chronic interstitial damage of this pathology.
Clinical presentation and diagnosisThe patient went to the emergency room due to poor general condition, hypotension, and dehydration. In the blood analysis showed: hemoglobin 13.1 g/dl, hematocrit 32.6%, glucose 109 mg/dl, urea 89 mg/dl, creatinine 2.88 mg/dl, sodium 108 mEq/l, potassium 2,0 mEq/liter, chlorine 64 mEq/L/liter, magnesium 1,2 mg/dl, uric acid 9,6 mg/dl, bicarbonate 30 mEq/liter, albumin 3,2 g/dl and calculated osmolality 238 mOsm/kg. Urine analysis reveals an osmolality of 230 mOsm/kg, potassium of 31 mmol/l, sodium of 25 mmol/l, and with fractional excretion of sodium of 1.36%. During admission, an immunity study was carried out, which was negative, common viral serologies and a proteinuria of 4,448 mg/day (albuminuria of 2,146 mg/day) was evidenced. A renal ultrasound was performed, which revealed kidneys measuring 9 cm with preserved parenchymal thickness, but with diffuse increase in echogenicity.
MeN typically affects middle-aged men with a history of agricultural work in hot and humid climates with an absence of a relevant medical history, such as high blood pressure or diabetes mellitus.2 In initial stages, MeN presents nonspecific symptoms such as asthenia, arthralgia, low-grade fever, dizziness, muscle cramps and / or subclinical rhabdomyolysis.22,23 The initial stages of the diseae are characterized by self-limited acute kidney damage that, perpetuated over time and ends up in CKD.24 The progression of CKD results in symptoms suggestive of renal involvement as dysuria, nocturia or lumbar pain.17 The failure to identify a kidney disease and the lack of access to diagnostic tests leads to medical management based on symptoms and in occasions the indiscriminate use of antibiotics or anti-inflammatory drugs, which increases the risk of CKD progression.25 The MeN disease mainly produces tubulointerstitial damage usually accompanied by ionic abnormalities as hyponatremia, hypokalemia, hypomagnesemia and hyperuricemia.21 The urine usually shows non-nephrotic range proteinuria, leukocyturia, isosthenuria, and urate crystals.17,26 All these alterations are reflected on a physical exam that is usually shows signs of volume depletion, with hypotension and orthostatism.27 In early stages the ultrasound reveals normal kidneys and as the disease progresses there are alterations of cortical echogenicity.28 Unfortunately, the lack of access to healthcare in most MeN endemic areas means that, not infrequently, patients consult in a situation of advanced CKD with uremic symptoms and urgent requirements RRT.
Therefore, the suspected diagnosis encompasses a series of clinical data that include progressive deterioration of renal function in the epidemiological context of farm workers in specific regions, with proteinuria without nephrotic syndrome and water electrolyte alterations typical of tubular interstitial damage. Currently, the accurate diagnosis requires a renal biopsy25 with histopathologic evaluation.
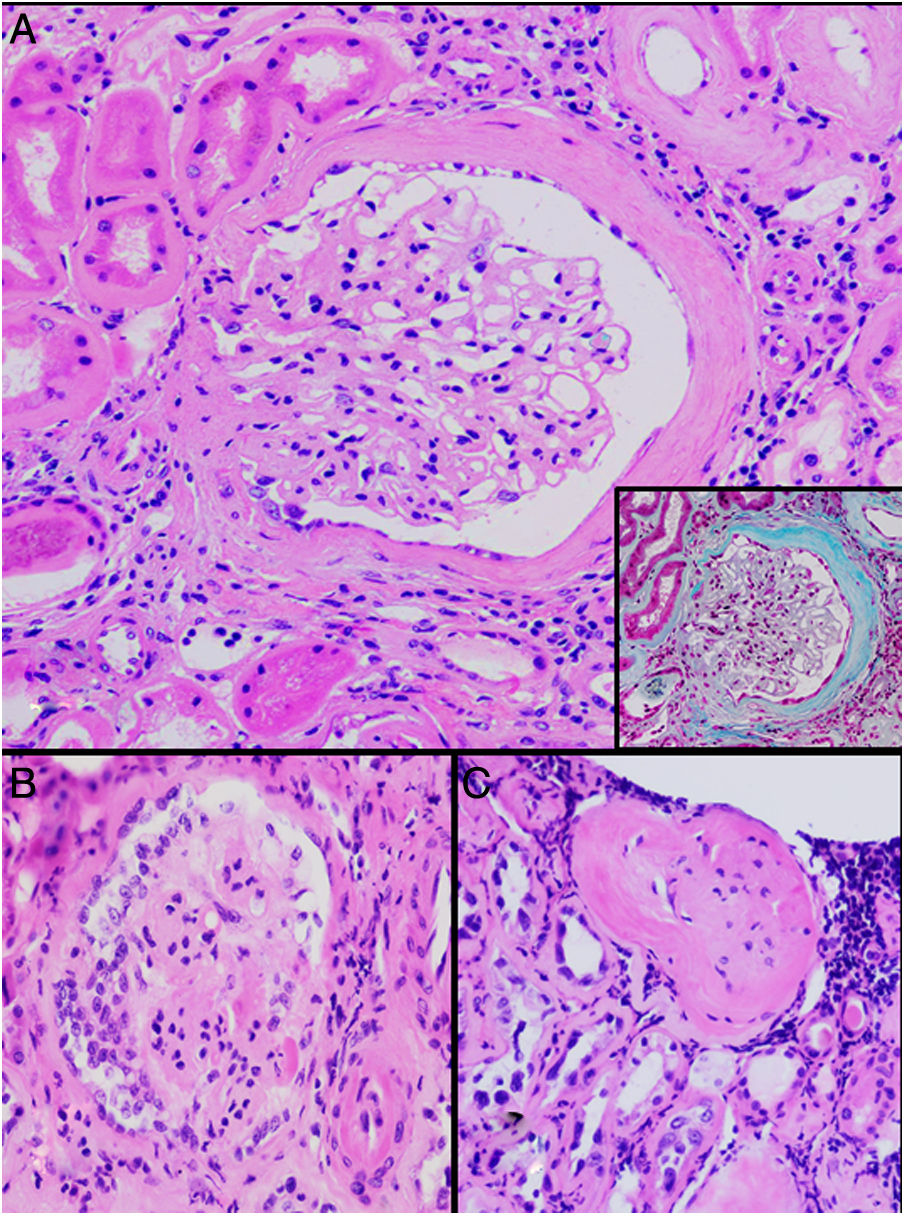
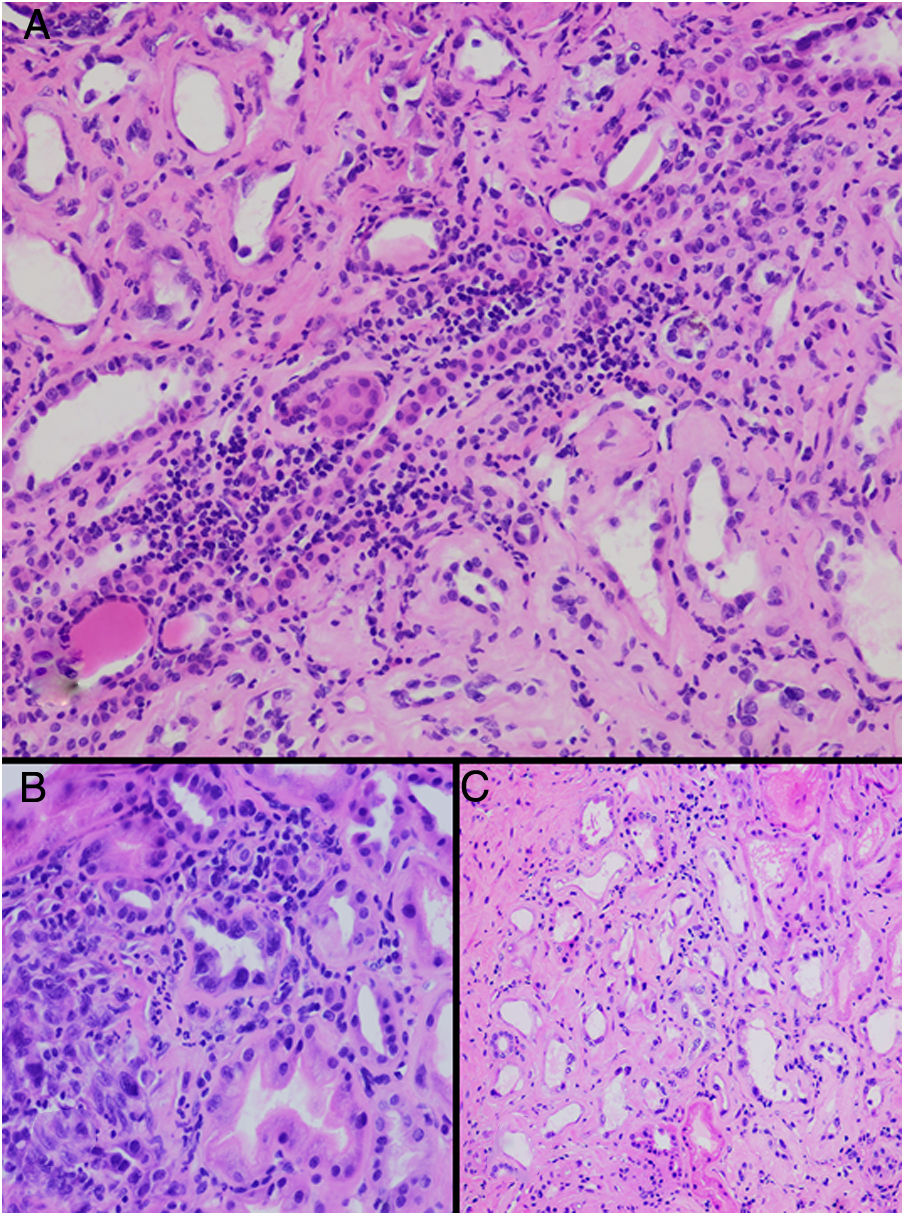
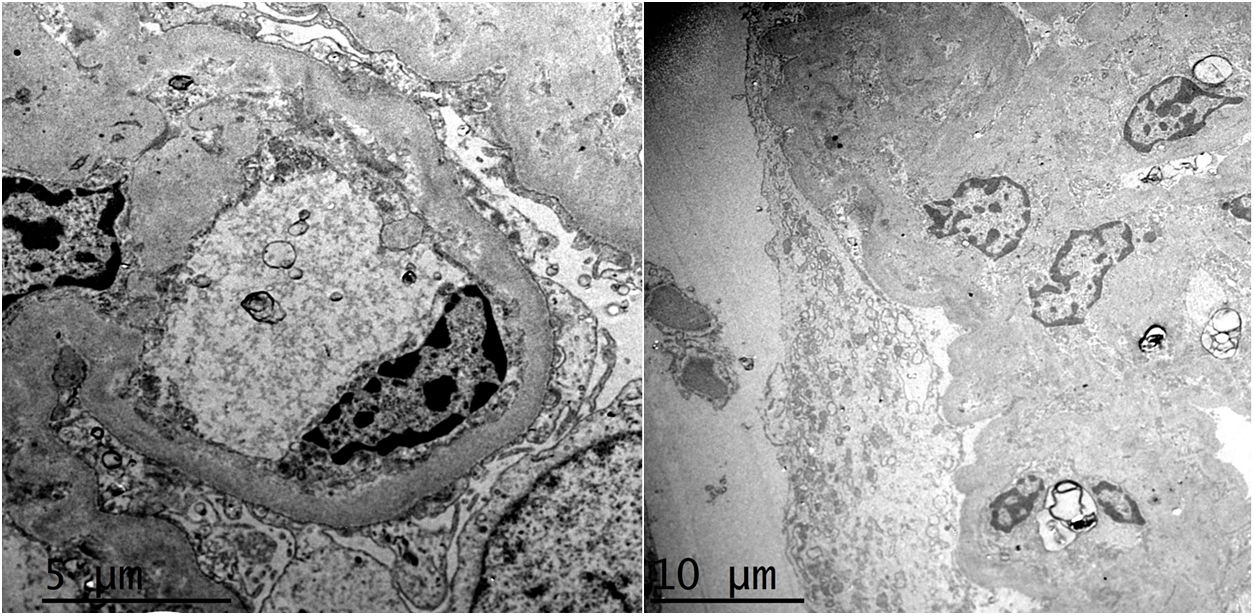
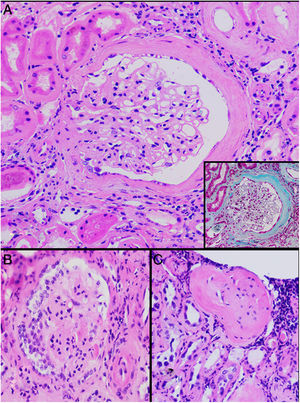
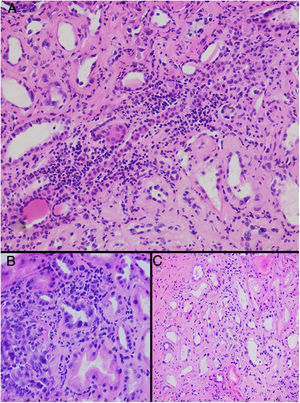
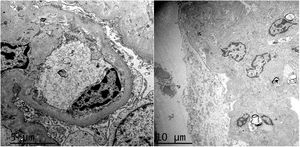
HistopathologyA renal biopsy showed global sclerosis in 60% of the glomeruli. The rest of the glomeruli, in a patchy distribution, presented areas with increased mesangial matrix, focal hyalinosis, mesangial hypercellularity, visceral podocyte hyperplasia, and pericapsular fibrosis (Fig. 3). Endo or extracapillary proliferation, fibrinoid necrosis, hyaline thrombi, wire loops, double contour, or “spikes” were not identified. Sixty percent of the tubules had epithelial atrophy, although some tubules showed ischemic damage with loss of epithelial cells, cell regeneration changes, and neutrophil infiltration (tubulitis). It was observed interstitial fibrosis (40%) and chronic inflammatory infiltrate (Fig. 4). Direct immunofluorescence was negative for anti IgM, IgG, IgA, C3, C1q, kappa and lambda light chains, and fibrinogen. In the electron microscopy evaluation, there were 6 of 7 globally sclerosed glomeruli and the only glomerulus that could be evaluated presented pericapsular fibrosis, Bowman’s space dilation, areas of sclerosis and other areas with signs of ischemia. The podocytes were partially assessable because they presented artifacts of preservation and some showed variable fusion that was non-significant. There were no parietal or mesangial deposits (Fig. 5).
Up to date the histopathological findings published are based on small series of clinical cases. The illustrative articles published by Wijkstrom et al. show a predominant and universal tubulointerstitial involvement with varying degrees of inflammation and chronicity.25,27 Although the early detection of MeN has been a diagnostic challenge, Fischer et al. have been able to made a histological description of the earliest stageof renal lesions. In a study including 11 native Nicaraguan patients who were followed prospectively, microscopy data confirmed that the renal histological lesions were tubulointerstitial nephritis. In addition, for the first time they describe that, accompanying the already known chronic lesions, in the initial stages there was also a component of acute inflammation with T lymphocytes and monocytes at the corticomedullary junction and infiltration of neutrophils in the tubular lumen, as well as interstitial edema. Furthermore, the authors describe glomeruli with architecture fully preserved, suggesting that interstitial involvement as the primary lesion at the renal level.26 The clinical translation of these histological findings is complemented by the clinical study performed in Nicaragua in which 326 sugar workers without previous disease were prospectively evaluated. In this group of patients, 34 workers presented acute deterioration of kidney function during occupational exposure, and of these, up to 50% developed CKD after one year.29
Glomerular alterations in the clinical case. (A) Glomerulus with pericapsular fibrosis and slight increase in the mesangial matrix (pericapsular fibrosis and perihilar sclerosis are highlighted in detail with Masson's stain) (H & E, × 400). (B) Glomerulus with diffuse sclerosis, decrease in the size of the glomerulus, and “embryonal hyperplasia” (H & E, × 400). (C) Enterely sclerosed glomerulus surrounded by lymphocytic infiltrate (H & E, × 200).
Tubulointerstitial alterations in the clinical case. (A) Marked lymphocytic infiltrate in the interstitium, accompanied by tubular atrophy (H&E, × 200). (B) Interstitial infiltrate with a predominance of lymphocytes with some neutrophils causing tubulitis, and marked tubular regenerative changes (H&E, × 400). (C) Zones of interstitial fibrosis with marked tubular atrophy (H&E, × 100).
Regarding glomerular involvement that occurs late, the changes are characterized by different degrees of global sclerosis, thickening of Bowman’s capsule and/or periglomerular fibrosis, folding of the glomerular basement membrane, and slight thickening of the mesangial matrix without proliferation cellular, as well as signs of chronic glomerular ischemia. Classically, immunofluorescence is negative.25,27
Ultrastructurally, in MeN there is podocyte alterations (consisting of segmental loss of pedicels and inclusions of vacuoles in the cytoplasmi, lipid droplets and bodies similar to lipofucsin) and signs of tubular atrophy with mild and nonspecific vascular changes.25,27 Most recently, an intriguing study has suggested lysosomal lesions in the proximal tubule very similar to those found in patients with nephrotoxicity by anticalcineurinics suggesting a common pathogenic pathway.30
Therefore, the histopathological findings of MeN originate from involvement at the tubulointerstitial level and, secondarily, it evolves to glomerular and vascular alterations.
Prevention and treatmentDuring the first hours and given the alterations in electrolytes, the patient requires intense hydroelectrolytic replacement, for which a central line was cannulated. At discharge, the patient was prescribed oral supplements of potassium, magnesium, and sodium chloride.
Given the pathogenesis of MeN, prevention is undoubtedly the best treatment. Thus it is essential to avoid the situations that elicits the disease such as dehydration and environmental exposure to certain substances. Access to water and avoid hours of maximum heat have been shown to be effective in this disease.31 In relation to exposure to toxins, although a causal relationship has not been demonstrated with most of the products used in agricultural communities, its indiscriminate use could enhance kidney damage, so adequate protection is recommended. In addition, as in any situation of volume depletion, it is important to avoiding drugs or substances that modify renal hemodynamics (anti-inflammatories, blockers of the renin-angiotensin-aldosterone system) or have been shown to nephrotoxic (antibiotics, herbal products).1,23,32,33 In addition, since MeN produces progressive CKD and without no specific treatment, early diagnosis with population screening, especially in risk areas, should be a priority.
Addressing the hypothesis that relates kidney damage to increased uric acid production, it would be advisable to avoid sugary and alcoholic beverages, thus controlling the load of fructose and the activation of pro-inflammatory mechanisms.34
Once the kidney damage is established, the main focus of the treatment is to reduce the negative consequences, mainly the alterations of water and electrolytes. This includes the use of supplements of: potassium (through diet and/or oral solutions), magnesium and, of course, sodium and water. In situations of refractory hypokalemia due to excessive interstitial damage, some authors even advocate the use of low-dose potassium-sparing diuretics.35
As for treatments of a more experimental nature, most of them have only been tested on animals. Based on the hypothesis of heat stress that includes an increase in uric acid production, Sánchez-Lozada et al. showed in a murine model that treatments aiming at reduce hyperuricemia help to reduce oxidative stress and the activation of the inflammasome.36 Roncal-Jiménez et al. analyzed the effect of allopurinol, a xanthine oxidase inhibitor, in mice that had been subjected to recurrent heat exposures. The results demonstrated a benefit in the renal function in mice on allopurinol, a protective effect that was correlated with the intrarenal concentration of uric acid.37
Despite the fact that patients tend to present metabolic alkalosis due to volume contraction, the use of bicarbonate has shown to be useful in MeN.36 It should be remembered that the basic etiological factors of MeN can trigger episodes of rhabdomyolysis, in which alkalinization of the urine plays a very important protective role, in addition bicarbonate favors the solubility of urate crystals.38 Furthermore, the correction of metabolic acidosis in advanced stages of CKD has been shown to slow down further progression of CKD.39
The use of angiotensin-converting enzyme inhibitors and angiotensin-II receptor blockers is controversial, since these are patients at high risk of AKI secondary to dehydration, and there are no specific studies to support their use. However, in a stable situation and extrapolating the antiproteinuric effect of these drugs, it could be considered with a very careful titration.40
Finally, the use of anti-inflammatory therapies, especially in the initial stages, could have some beneficial effect to reduceof acute inflammatory infiltrates, although it is true that to date no specifically treated case has been reported in MeN, probably due to late diagnosis of the disease. In fact, the presence of leukocytosis has been established in some specific work as a predictor of good renal prognosis.41 Anecdotally, the use of anti-oxidants (vitamins C and E) has been proposed as possible treatments in NeM.42
The prognosis for MeN is poor, with high rates of progression to advanced CKD and with significant mortality. The factors affecting poor evolution probably are related with the late diagnosis of the disease and in the lack of access to specialized health resources, although it is true that small series of cases have shown that other determinants (some modifiable) such as anemia, paresthesia or lack of breaks during the work may contribute negatively to the development of this disease.41,43,44 The patient was followed up in the nephrology outpatient clinics, and required numerous admissions due to volume depletion and deterioration of renal function. Progressively, CKD reached stage G5, for which hemodialysis was started through an arteriovenous fistula.
In conclusion, MeN is a chronic tubulointerstitial disorder, of undetermined origin with a multifactorial etiology based on dehydration with volume depletion in workers exposed to environmental toxins. Although we lack specific therapeutic measures, the prevention of episodes of excessive heat and the management of water and electrolyte alterations are the basis of its management.
FinancingThe present study has not received any type of funding.
Conflict of interestsThe authors have no conflict of interest.