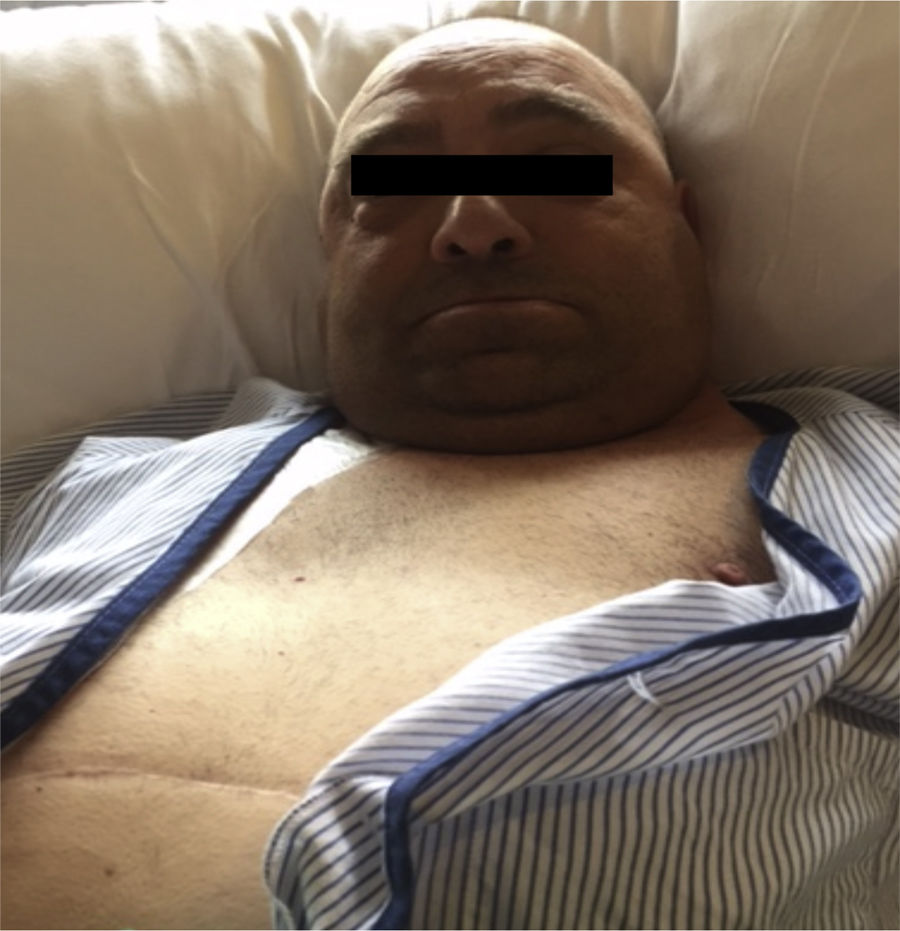
Rubinstein-Taybi syndrome is a systemic disease with variability in its clinical expression. The incidence in the general population is 1/300,000–1/720,000 inhabitants. Mutations have been described in the gene that encodes the transcriptional coactivator binding protein (CREB), mapped in 16p13.3; it is a nuclear protein that participates as a coactivator in gene expression regulated by cyclic AMP. Approximately 25% of the patients diagnosed have a microdeletion in the 16p13.3 region demonstrable by in situ hybridization techniques (FISH).1–3 It is common for patients to have microcephaly and short stature, a characteristic face (Fig. 1). Other manifestations include hirsutism, chronic gastritis and/or gastroesophageal reflux, cardiac and neurological defects, short stature, ptosis, low implantation ears, colobomas and polydactyly. At the renal level, they may present with renal agenesis, horseshoe kidney, the presence of vesicoureteral reflux, nephritic colic, megaureter, among others. They may present with chronic kidney disease, and with time they need renal replacement therapy.4–8
Gastrointestinal discomfort is not uncommon. This is due to reflux and constant food transgressions. Although it is true that they are patients with a higher risk of presenting neoplasms, neuroendocrine tumors are not the most frequent9 and the clinical picture can be imperceptible, making diagnosis difficult.
We present the clinical case of a 46-year-old male diagnosed with Rubinstein-Taybi syndrome in a chronic hemodialysis program since 19-08-2007. Previously, the patient was in a peritoneal dialysis program from 2004 to 2007, requiring a change of technique in 2007 due to repeated bacterial peritonitis. Kidney transplant from a cadaver donor in June 2010, not functioning due to arterial thrombosis and transplantectomy performed in July 2010. Multiple episodes of self-limiting abdominal pain not necessary associated with frequent admissions, recurrent UTIs that were accompanied on several occasions by constipation, diarrhea and sometimes vomiting. At the renal level, he presented right lower segmental megaureter, ureterohydronephrosis with right ureteral lithiasis. Multiple episodes of renal lithiasis. Left renal hypoplasia. Episode of renal lithiasis in February 2009, assessed by urology that placed double J, with catheter removal on 01-14-2010. A lithotripsy was performed in 2009 at the University Hospital of León with dubious disappearance of ureter dilation in subsequent controls. The surgical history included an inguinal herniorrhaphy, left inguinal hernioplasty, extirpation of the left testis, catheter placement for PD in 2004 and removal in 2007. Kidney transplant in 2010. Ureterocelectomy in September 2012.
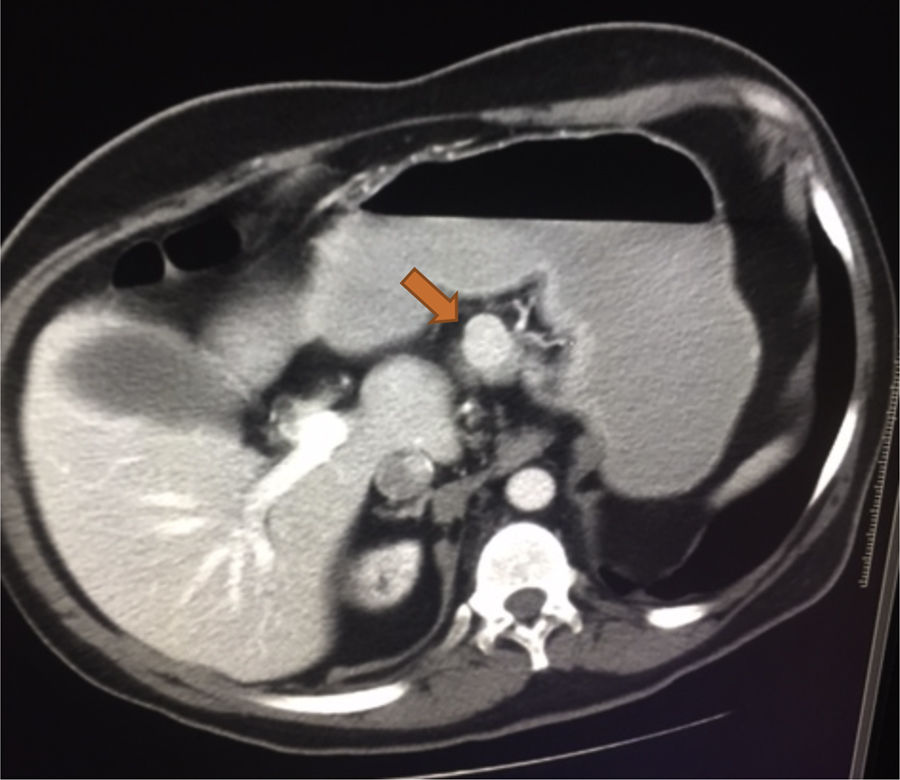
He was admitted to the GI service from 07-10-2011 to 20-11-2011 due to a nonspecific abdominal pain. During the admission, he presented repeated vomiting, subsequent tests show the presence of chronic pancreatitis, initiating enzymatic treatment with slight clinical improvement. Continues with digestive pain, marked anxiety, much emotional liability that made the communication and physical examination difficult. It should be noted that the gastrointestinal symptoms were so unspecific that sometimes improved with psychological support from nursing, NSAIDs, antiemetics or anxiolytics. The persistence of nonspecific abdominal pain, especially during hemodialysis sessions, suggests an underlying disease not yet diagnosed. An abdominal ultrasound was performed, with no relevant findings, except for previously known kidney disease. Abdominal CT was requested, showing a dubious mass of 2cm protruding in the upper edge of the body of the pancreas near the isthmus, as well as a Bochdalek hernia.
The patient was referred to the surgery and endocrinology service and was diagnosed with a probable neuroendocrine tumor. As a treatment, a subcostal exploratory laparotomy was performed, observing a highly vascularized mass 2.5cm in diameter without apparent contact with the Wirsung canal (Fig. 2). Stability of the lesion and Ki-67 less than 2% is appreciated, so it is decided to enucleate the tumor that was performed without any incidents. The anatomopathological diagnosis showed a mass in the body of the pancreas of 2cm in diameter, G1 of the 2010 WHO classification, which respects the surgical border, although it is located at less than 1mm from surgical border. Chromogranin to isintaphysin: (+) Ki-67: low rate of cell proliferation (−1%). Lymph node in greater curvature, adipose tissue without histological alterations. Peritoneal solitary cyst. The patient remained stable in the postoperative period, being transferred to the internal medicine for recovery.
The syndrome of Rubinstein-Taybi in childhood is a difficult diagnosis. It should be differentiated from other microdeletions such as Floating-Harbor syndrome and Cornelia de Lange syndrome. They can present a great clinical variability. Emotional liability and cognitive deficit make the clinical examination in these patients more complex, with greater need to rely in complementary tests in order to reach the diagnosis.
Please cite this article as: Romaniouk I, Romero A, Runza P, Nieto C, Mouzo R, Simal F. Manejo de tumor neuroendocrino en paciente con el síndrome de Rubinstein-Taybi en hemodiálisis crónica. Nefrologia. 2018;38:446–448.