La hipercalcemia postrasplante debida a la persistencia del hiperparatiroidismo (HPT) secundario tiene una prevalencia elevada en los primeros 3 meses postrasplante, que va disminuyendo a lo largo del primer año, aunque en torno al 5-10 % de los trasplantados renales persiste en el tiempo. La mayor resorción ósea y la mayor reabsorción tubular de calcio debido a la acción de la hormona paratiroidea (PTH) parecen ser los mecanismos principalmente implicados en la hipercalcemia. La PTH en el momento del trasplante renal (TR) es el factor que determina el desarrollo de hipercalcemia pos-TR, aunque a veces se encuentre enmascarada en los pacientes bien controlados con tratamiento médico. Cada vez más los pacientes en diálisis reciben cinacalcet como tratamiento del HPT secundario. La retirada del calcimimético en el momento del trasplante renal da lugar a una mayor prevalencia de hipercalcemia e hiperparatiroidismo en estos pacientes. En los pacientes con PTH bien controlada con cinacalcet antes del trasplante, existe una relación directa entre la dosis y el desarrollo posterior de hipercalcemia, probablemente porque indica la presencia de un HPT secundario más severo. La hipercalcemia puede tener efectos deletéreos sobre el injerto renal dando lugar a calcificación tubulointersticial. La hipercalcemia persistente es una marcador de aumento del riesgo de empeoramiento de la patología ósea de estos pacientes. Hoy en día, la primera opción de tratamiento la constituye el cinacalcet, y ante la ausencia de respuesta se valorará la realización de una paratiroidectomía. En esta revisión proponemos un algoritmo de manejo de la hipercalcemia pos-TR.
Post-transplant hypercalcemia due to persistent secondary hyperparathyroidism (HPT) has a high prevalence in the first 3 months after surgery and decreases during the first year, but it persists over time in around 5-10% of renal transplant patients. The increased bone resorption and tubular reabsorption of calcium due to the action of the parathyroid hormone (PTH) appear to be the main mechanisms involved in hypercalcemia. At the time of the renal transplantation (RT), PTH is the factor that determines the development of post-RT hypercalcemia, although it is sometimes masked in patients who are well controlled with medical treatment. The number of dialysis patients receiving treatment with cinacalcet for secondary HPT is increasing. The withdrawal of the calcimimetic at the time of renal transplantation results in a higher prevalence of hypercalcaemia and hyperparathyroidism in these patients. In patients with PTH well controlled with cinacalcet before transplantation, there is a direct relationship between the dose and the subsequent development of hypercalcemia, probably because it indicates the presence of a more severe secondary HPT. Hypercalcemia may have deleterious effects on the renal graft, resulting in tubulointerstitial calcification. Persistent hypercalcemia is a marker of an increased risk of bone disease deterioration in these patients. Nowadays, the first treatment option is cinacalcet and if there is no response, we consider performing a parathyroidectomy. In this review, we propose an algorithm for management of post-RT hypercalcemia.
INTRODUCTION
Hypercalcemia is frequently found in patients with a functioning renal allograft, with prevalence ranging between 5% and 66% according to the series,1-4 although severe hypercalcemia (total calcium > 12mg/dl) is quite exceptional.
These differences in prevalence are due to various factors, such as the different cut-off values considered for diagnoses of hypercalcemia or the value considered as serum ionised calcium or total calcium, corrected or not by albumin.
Another factor to bear in mind is the period considered, since the prevalence of hypercalcemia decreases over time after transplant (RT). In general, hypercalcemia prevalence has been reported as being much higher during the first three months following RT, but it decreases progressively in the first year and remains practically stable at around 5-10% thereafter.2-5
Until the introduction of the management guidelines for mineral and bone metabolism disorders in patients with chronic kidney disease in 2003,6 the series of patients compiled showed a higher incidence and prevalence of post-RT hypercalcemia. In fact, in 1973, hypercalcemia prevalence above 30% was reported one year after the RT, which persisted practically unchanged 2 and 5 years after transplantation.5
Following the introduction of the guidelines and their clinical application, there appeared to be a turning point in the prevalence of post-transplantation hypercalcemia. This was the case when patients studied between 2004 and 2006 were compared to a hostoric control group of patients studied between 1989 and 2002,1,2 in which we observed a 41% prevalence of hypercalcemia in the historic group, while in the more recent group, it had been reduced to 14%.
Nevertheless, most studies on post-RT calcium metabolism were carried out prior to the introduction of cinacalcet for the treatment of secondary hyperparathyroidism (HPT) in dialysis patients.
Cinacalcet was approved by the United States Food and Drug Administration in 2004 and by the European Medicines Agency in 2005 to treat secondary HPT in dialysis patients, and subsequently, to treat parathyroid carcinoma and primary HPT. The efficacy of cinacalcet for controlling the parathyroid hormone (PTH) in dialysis patients has been widely demonstrated.7-10
The better control of secondary HPT in dialysis patients after the introduction of cinacalcet probably has an effect on mineral and bone metabolism disorders after RT, although few studies have been carried out in this regard.
CAUSES OF HYPERCALCEMIA
Studies from the era prior to the introduction of cinacalcet showed that high serum PTH values were the main predictor of post-transplantation hypercalcemia.1
The introduction of cinacalcet has resulted in a better control of secondary HPT, and as such, there has been a decrease in the percentage of patients who undergo RT with high serum PTH values. However, withdrawing cinacalcet after RT results in high PTH levels immediately after transplantation, and consequently, hypercalcemia in a high number of patients.11
In a recent study, although carried out on a small number of patients, this was also observed and patients on treatment with cinacalcet before transplantation whose treatment was discontinued on the day of surgery displayed a higher hypercalcemia incidence (corrected Ca >10.3mg/dl) three months after transplantation than those patients who had not received cinacalcet (42.9% vs. 11.4%) .12
In our experience, when we compared patients who received cinacalcet at the time of RT and those who did not receive it, we found a hypercalcemia proportion (corrected Ca > 10.3mg/dl) of 26.3% 3 months after transplantation compared with 0% hypercalcemia in the control group (P=.01), despite both groups displaying similar PTH and calcaemia values at the time of transplantation.13
Studies subsequent to the introduction of cinacalcet showed that pre-transplantation PTH only influenced post-transplantation Ca values in patients who were not on treatment with cinacalcet, while, in patients who received cinacalcet prior to RT, it was the cinacalcet dose that predicted the subsequent development of HPT and hypercalcemia, being a marker for the severity of secondary HPT in these patients.11,12
PHYSIOPATHOLOGY
The physiopathological mechanisms suggested as responsible for post-transplantation hypercalcemia are:
- More tubular calcium reabsorption, due to PTH action. The results of different studies are disparate; while some show a decrease in fractional calcium excretion,1 others refer to greater urinary calcium excretion.3 It seems that the effect of PTH increasing tubular calcium reabsorption would be more evident in the long term and less evident immediately after transplantation.1,3
- More intestinal calcium absorption, due to increased serum calcitriol caused by the increase in its synthesis due to PTH stimulation. Serum calcitriol values gradually recover in most patients after RT and we have observed that this is in relation to the rapid and progressive decrease in serum FGF23 (fibroblast growth factor 23) values.13 Nevertheless, no study has shown differences in calcitriol values between patients with hypercalcemia and normocalcemia.1,2
- More bone calcium resorption, mediated by the PTH. This seems to be the mechanism involved particularly in recent RT. In patients with hypercalcemia, we observed significantly higher serum alkaline phosphatase values than in normocalcaemic patients, which suggests an increased bone turnover.1
CONSEQUENCES OFHYPERCALCEMIA
- Effect on the kidney graft. Hypercalcemia, through a vasoconstriction mechanism may impair renal graft function, both acutely and chronically.3,14 It may also cause tubulointerstitial calcifications that may have a negative influence on long-term graft survival.15
- Other effects. Cases of pancreatitis have been reported in
renal transplant patients with hypercalcemia due to HPT,16 and it has also been demonstrated that it increases the risk of soft tissue calcification and the development of vascular calcification.17
Hypercalcemia is fundamentally the result of an increase in bone remodelling with increased calcium reabsorption, and as such, persistent hypercalcemia will indicate that there is an increased risk of bone disease deterioration in these patients.16,18
MANAGEMENT OF POST-TRANSPLANTATION HYPERCALCEMIA
To manage hypercalcemia due to persistent HPT following RT, the alternatives are:
a) Watchful waiting. Initial action, in the case of moderate hypercalcemia may be to monitor serum calcium and PTH values while waiting for them to normalise over time. However, when there is significant hypercalcemia (> 11mg/dl) and/or it persists over time (> 1 year), or it is symptomatic, we should take a more proactive approach.
In our opinion, shared by others,19 in the absence of symptoms or significant hypercalcemia, it would be prudent to wait at least a year for spontaneous resolution, although some authors advocate waiting only 3-6 months after RT before opting for parathyroidectomy.20
b) Parathyroidectomy. Traditionally, it was the only alternative for controlling hypercalcemia in these patients and it remains the best option for correcting hypercalcemia when compared with calcimimetics.21 However, it carries an increased risk of acute and chronic hypocalcaemia,22 as well as a high rate of persistence or recurrence of HPT, depending on the technique used.
Parathyroidectomy is not without risks:
- Hungry bone syndrome, which results in severe hypocalcaemia following surgery, with symptoms ranging from paraesthesias to marked tetany, which is sometimes difficult to control, requiring high doses of calcium and vitamin D supplements.
- Local surgical complications: surgical wound infections, recurrent nerve palsy (reported in 1% of surgeries with subsequent progressive recovery).
- Deterioration in renal function. Some studies have reported a deterioration in renal function following parathyroidectomy,21,23-26 while others have not reported it.19,22 The deterioration in renal function is independent of the type of parathyroidectomy and it appears that patients who experience a deterioration in renal function already have deterioration prior to parathyroidectomy.27
The surgical options are:
- Total parathyroidectomy with autotransplantation in the forearm. A total parathyroidectomy with autotransplantation in the forearm results in quicker calcemia correction; however, there is a higher risk of hypocalcaemia.
In patients with a functioning RT, the recurrence rate appears to be similar for this technique and subtotal parathyroidectomy.28
In the event of recurrence/persistence, it is sometimes difficult to discern whether this is due to a hyperplasia of the autotransplant or residual tissue in the cervical area. In this case, mibi scintigraphy may help us.29
The gland autotransplanted in the forearm in the event of recurrence seems to be more accesible,30 but, in the case of significant hyperplasia (also called parathyromatosis) it usually spreads in the implant area and is normally very difficult to remove.
This involves the removal of all the parathyroid glands except for the remainder of a well-vascularised gland the size of a normal gland. The remainder of the gland that is left should have a normal macroscopic appearance or that of a simple diffuse hyperplasia.
- Many authors advocate this technique as they consider it to be the least aggressive one.28
- Total parathyroidectomy without autotransplantation. This is an effective alternative in terms of improving bone pain and it is probably the technique in which we observe the lowest number of recurrences/relapses of HPT.31-34
In contrast, most of these patients usually require calcium and vitamin D supplements indefinitely in order to avoid hypocalcaemia and osteomalacia, respectively.
- Selective parathyroidectomy. This involves performing a parathyroidectomy on only one or two parathyroid glands. If with the cervical ultrasound examination and/or MIBI scintigraphy it is shown that there are one or two adenomatous glands, some authors advocate removing only those pathological glands (enlarged and/or with increased uptake).
With this technique, the acute hypocalcaemia percentage is less than with the previous techniques and the percentage of persistent hypocalcaemia is almost nil.16,35 By contrast, it appears to increase the risk of persistence/recurrence of HPT,28,36 although there are also series that show good progression and low incidence of recurrence.16,35,37
c) Calcimimetics. Since 2006, calcimimetics have been employed as an alternative treatment for hypercalcemia secondary to persistent secondary HPT (also called tertiary HPT) in patients with a functioning RT.38-46
In all published studies, the appropriate control of calcemia was confirmed with an improvement in serum PTH and phosphatemia.
In the majority of these studies, effects on renal function were not reported, although in some exceptional cases a slight deterioration in renal function after three months44 or after one year on treatment with cinacalcet41 was reported, although in this latest study, patients with a deterioritation in renal function were included prior to starting cinacalcet, which was reversible in all cases after medication was discontinued.41
Although the cause of this occasional deterioration in renal function is not clear, it has been suggested that it could be related to a decrease in PTH values, as with parathyroidectomy.23,47 This has already been described in previous experimental studies that suggested that PTH plays a role in the regulation of renal perfusion and mesangial cell function.48,49
Another possibility that has been suggested is that the deterioration in renal function could be related to hypercalciuria secondary to the decrease in PTH; nevertheless, in most studies of patients being treated with cinacalcet we did not observe significant hypercalciuria and developments of renal tubular calcification was not observed in any case.50
Recently, a retrospective study comparing the use of calcimimetics with parathyroidectomy and medical observation without any surgery in renal transplant patients with stable renal function and tertiary HPT observed a higher rate of acute renal failure and a deterioration of the renal graft in the group of patients who did not receive treatment for their tertiary HPT.22
Cinacalcet is a safe therapeutic option for treating hypercalcemia in patients receiving a RT; nevertheless, recurrence of HPT is observed after the withdrawal of calcimimetics.38,43
Currently, there is no marker that could indicate the suitable time point to withdraw cinacalcet treatment.
Considering that it is a group of patients that is often treated with multiple drugs and that maintaining this medication for an indefinite period incurs a high economic cost, it is necessary to carry out studies that allow us to find a marker that helps us know at what time we can withdraw calcimimetic treatment without the risk of recurrence of HPT and the resulting development of hypercalcemia.
After all, in our opinion, treatment with calcimimetics must be tested for the management of hypercalcemia after renal transplantation before performing a parathyroidectomy
PROPOSAL FOR MANAGING POST TRANSPLANTATION HYPERCALCEMIA
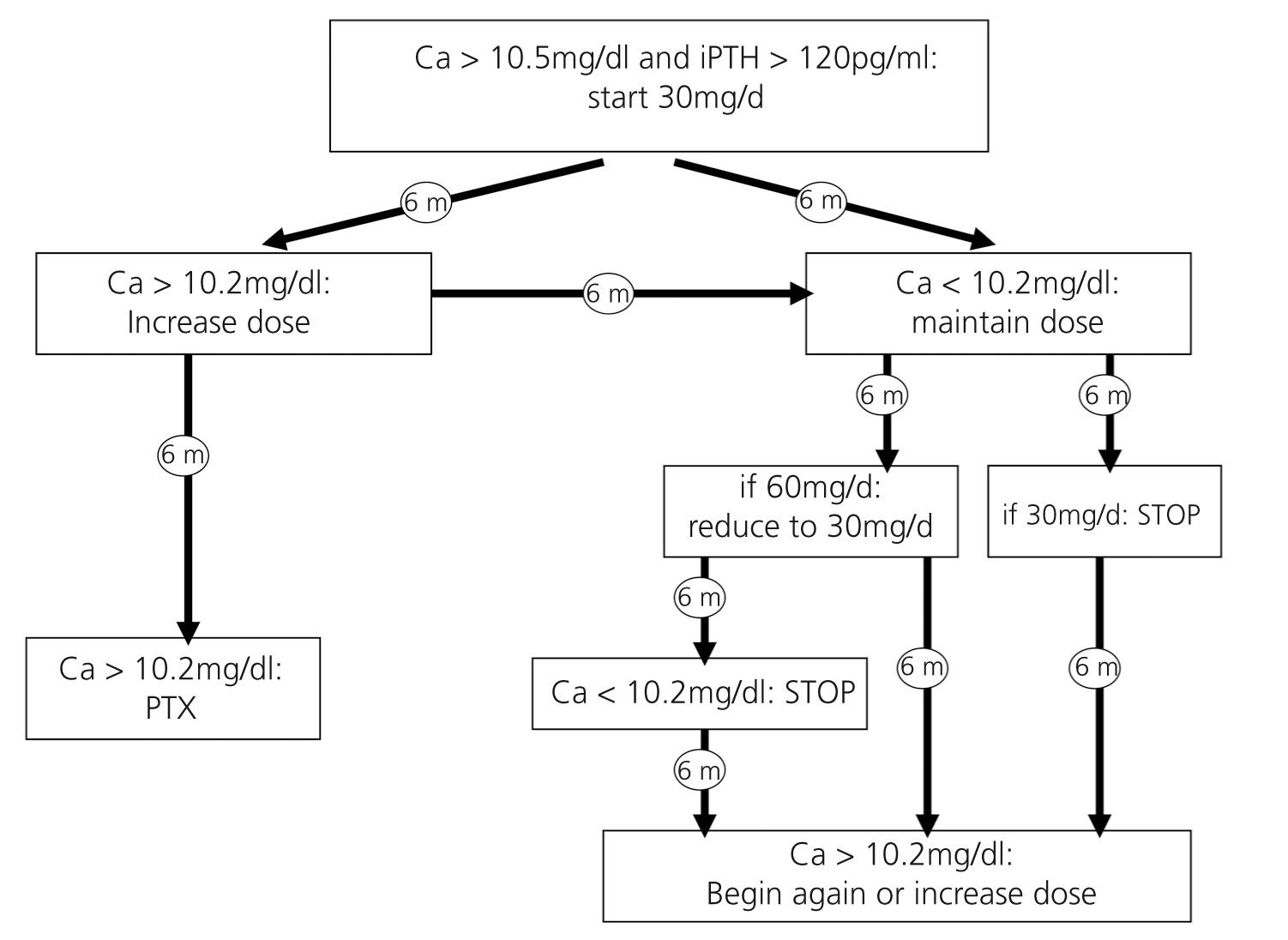
Upon reviewing the literature and taking into account accumulated clinical experience, we offer a proposal for managing hypercalcemia after renal transplantation, set out schematically in the form of an algorithm (Figure 1).
We propose beginning treatment with cinacalcet in all patients with Cac > 11mg/dl and in those with Cac between 10.5 and 11mg/dl for more than 6 months, in all cases with PTH > 120pg/ml. The starting dose will be 30mg/day of cinacalcet, which will be maintained for 6 months.
In the event that the patient responds to the initial dose of 30mg/day (Cac < 10.2mg/dl 6 months after treatment), we propose maintaining the same dose for another 6 months (1 year total) and, if there is good control, discontinuing the medication, with a new test after 6 months, and if calcaemia increases again above 10.2mg/dl we would recommence treatment with cinacalcet.
If, on the contrary, with an initial dose of 30mg/day calcaemia remains high (Cac > 10.2mg/dl after 6 months), we would increase the dose to 60mg/dl. If by increasing the dose we are able to control calcaemia, we could consider decreasing the dose again to 30mg/day after 6 months and maintaining this regimen for at least another 6 months. If calcaemia is controlled in this way, we could consider withdrawing the drug.
In the event that the patient has Cac > 10.5mg/dl for more than 12 months despite an increased dose of cinacalcet, the most suitable course of action would probably be to consider a parathyroidectomy.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Proposal managing of hypercalcemia after renal transplantation.