Introducción: A diferencia de los pacientes tratados con diálisis peritoneal, la programación de una dosis incremental de diálisis no se considera en el enfermo tratado con hemodiálisis (HD) periódica, ni tampoco es habitual tener en cuenta la función renal residual en el cálculo de la dosis total de diálisis, asumiéndose como tal la proporcionada exclusivamente por el aclaramiento del dializador. A partir del año 2006 decidimos establecer una pauta incremental de diálisis al inicio del tratamiento renal sustitutivo, valorando la posibilidad de comenzar con 2 HD/semana cuando el aclaramiento renal de urea fuera igual o superior a 2,5 ml/min. En el presente trabajo presentamos nuestra experiencia de los primeros 5 años de aplicación de esta pauta incremental de HD y su repercusión sobre la función renal residual. Metodología: Se han incluido a todos los enfermos que iniciaron tratamiento con HD periódica entre el 1/1/2006 y el 30/9/2010, y permanecieron en diálisis más de tres meses. El seguimiento de los enfermos finalizó el 31/12/2010 (fecha de cierre del estudio). Cuando un enfermo inicia HD se determina el aclaramiento de urea y creatinina con las concentraciones de urea y creatinina en una muestra de sangre obtenida antes de la diálisis y la orina de las 24 horas previas al inicio de la primera sesión de diálisis de la semana. Si el aclaramiento de urea es igual o superior a 2,5 ml/min, se comienza con 2 HD/semana, siempre que lo permita la situación clínica a criterio del médico responsable. La función renal residual se analiza cada dos meses hasta que la diuresis es inferior a 100 ml/día, que se considera nula. Evaluamos el descenso de la función renal residual, calculando el ritmo de descenso del filtrado glomerular (ml/min/mes) y de la diuresis de 24 horas (ml/mes) en los pacientes que se dializan con 2 y 3 HD a la semana. En enero de 2010 realizamos un corte transversal en el que se relacionó el filtrado glomerular con diversos parámetros clínicos y analíticos en pacientes que se dializan 2 y 3 veces a la semana. Resultados: En el período de estudio, se incluyeron 95 pacientes, de los cuales 41 (43%) empezaron con 2 HD y 54 (57%) con 3 HD a la semana. El tiempo medio que permanecieron con la pauta de 2 HD/semana fue de 11,1 ± 7,2 meses (rango 2-25). De los 41 enfermos que comenzaron con 2 HD/semana, 10 recibieron el trasplante mientras permanecían con esta pauta, 1 fue transferido a diálisis peritoneal, 6 recuperaron función renal y pudieron abandonar el tratamiento con diálisis, 15 pasaron a la pauta de 3 HD/semana y 9 continuaban dializándose con la pauta de 2 HD/semana en el momento de cierre del estudio. De los 15 enfermos que pasaron a la pauta de 3 HD/semana, 4 fueron trasplantados, 3 fallecieron y los 8 restantes continuaban en HD en la fecha de cierre del estudio. El análisis de Kaplan-Meyer mostró que la supervivencia de los pacientes que iniciaron 2 veces/semana fue mayor (log-rank 3,964, p = 0,04). La pérdida del filtrado glomerular y de la diuresis de 24 horas fue menor en los enfermos con la pauta de 2 HD/semana: 0,22 ± 0,36 vs. 0,89 ± 1,26 ml/min/mes para el filtrado glomerular (p = 0,001), y 90,59 ±132 vs. 206,23 ± 286 ml/mes para la diuresis de 24 horas (p = 0,001). En el corte transversal realizado en enero de 2010, 17 pacientes estaban con pauta de 2 HD/semana y 47 con 3 HD/semana. La concentración sérica de β2-microglobulina fue significativamente menor en el grupo con 2 HD/semana (19,7 ± 5 vs. 38,3 ± 13, p = 0,000). La concentración media de hemoglobina fue similar en ambos grupos, con una dosis de eritropoyetina significativamente menor en los pacientes de 2 HD/semana (7058 ± 3749 vs. 12.553 ± 10.826 unidades/semana, p = 0,037). Conclusión: En una población seleccionada, el inicio de la HD se puede hacer de forma incremental, comenzando con dos sesiones de diálisis a la semana. En nuestra experiencia es una pauta segura que probablemente contribuye a preservar la función renal residual.
Introduction: In contrast to patients treated with peritoneal dialysis, those on periodical haemodialysis (HD) do not receive programmed progressive increases in dialysis dosage, nor is residual renal function taken into account in the calculation of the total dialysis prescription; rather, only dialyser clearance is factored into the equation. In 2006, we decided to establish a progressively increasing dialysis regimen at the start of renal replacement therapy, evaluating the possibility of starting with 2 sessions of HD/week when renal clearance of urea was equal to or greater than 2.5ml/min. This study summarises our experience during the first 5 years of application of this progressively increasing HD prescription and its repercussions on residual renal function. Methods: We included all patients who started periodical HD between 1/1/2006 and 30/9/2010 and remained on dialysis for more than three months. The follow-up period ended on 31/12/2010 (study end date). When a patient started HD, urea and creatinine clearance levels were measured based on urea and creatinine concentrations in blood samples taken before dialysis and in urine samples taken 24 hours prior to starting the first dialysis session of the week. If urea clearance was equal to or greater than 2.5ml/min, 2 sessions of HD per week were applied, as long as the patient’s clinical situation allowed for it (according to the criteria of the attending physician). Residual renal function was analysed every 2 months until diuresis was less than 100ml/day, which is considered to be basically null. We evaluated the decrease in residual renal function, calculating the rate of decrease in glomerular filtration (ml/min/month) and 24-hour diuresis (ml/month) in patients receiving 2 and 3 HD sessions per week. In January 2010, we took a cross-sectional sample, evaluating glomerular filtration and how this value was associated with various clinical and laboratory parameters in patients receiving 2 or 3 dialysis sessions per week. Results: During the study period, 95 patients were included in the study, 41 of which (43%) started with 2 HD sessions per week, and 54 (57%) with 3 sessions per week. The mean time that patients remained on the 2HD sessions/week regimen was 11.1±7.2 months (range: 2-25 months). Of the 41 patients that started with 2 HD sessions/week, 10 received a transplant while on the treatment regimen, 1 was transferred to peritoneal dialysis, 6 recovered renal function and were able to abandon dialysis treatment, 15 were switched to the 3 HD sessions/week regimen, and 9 continued on the 2 HD sessions/week regimen at the time the study ended. Of the 15 patients that were switched to the 3 HD sessions/week regimen, 4 received transplants, 3 died, and the remaining 8 continued on HD until the end of the study. A Kaplan-Meier survival analysis revealed that patients who started on the 2 HD sessions/week regimen had a greater survival rate (log-rank: 3.964; P=.04). Losses in both glomerular filtration rate and 24-hour diuresis were lower in patients on the 2 HD sessions/week regimen: 0.22±0.36ml/min/month vs 0.89±1.26ml/min/month for glomerular filtration (P=.001), and 90.59±132ml/month vs 206.23±286ml/month for 24-hour diuresis (P=.001), respectively. In the cross-sectional sample taken in January 2010, 17 patients were on the 2 HD sessions/week regimen and 47 were on the 3 HD sessions/week regimen. Serum concentrations of β2-microglobulin were significantly lower in the 2 HD sessions/week group (19.7±5 vs 38.3±13; P=.000). The mean haemoglobin concentration was similar between the two groups, with a significantly lower dose required of erythropoietin in patients on the 2 HD sessions/week regimen (7058±3749 units/week vs 12 553±10 826 units/week; P=.037). Conclusion: In select populations, the start of HD can be administered on a progressively increasing dosage, starting with two sessions/week. In our experience, this is a safe prescription that probably contributes to preserving residual renal function.
INTRODUCTION
Dialysis doses in chronic patients treated with peritoneal dialysis are calculated using the sum of peritoneal and renal urea clearance rates. The persistence of residual renal function allows for programmed progressive increases in dialysis doses, starting renal replacement therapy with a low dose of peritoneal clearance, which then is increased as renal clearance decreases.1,2 Residual glomerular filtration rate is an extremely relevant factor in these patients, and quantifying and maintaining these values is a very important component of this type of treatment.3,4
However, programmed increases in dialysis doses are not considered an option in patients treated with periodic haemodialysis. The normal frequency of intermittent dialysis as renal replacement therapy for chronic renal failure is three sessions per week (3 HD/week), which is instated at the beginning of a treatment plan.5,6 It is also not a common practice to take residual renal function into account when calculating total dialysis doses, and only the clearance provided by the dialyser is used.7,8
However, it is a high priority in any patient with chronic renal failure starting dialysis to preserve residual renal function, since this allows for better control of body volumes and ion concentrations such as potassium and phosphorous, as well as elimination of medium-sized molecules such as β2-microglobulin and protein-linked molecules that are difficult to extract using a dialyser.9-13 The loss of residual renal function varies from one patient to another, both on haemodialysis and on peritoneal dialysis, but certain characteristics inherent to haemodialysis, such as episodes of hypotension, volume depletion, and activation of inflammatory mediators associated with the biocompatibility of the dialyser and the dialysate, have been implicated as the primary causes of loss of residual renal function in these patients.14-16
In our department, it is common practice to measure diuresis and renal clearance of urea and creatinine at the start of haemodialysis treatment and every two months in patients that retain residual diuresis, and we consider renal clearance of urea as part of the dialysis dose that patients receive. In 1985, Gotch17 used the urea kinetic model to establish that an adequate dialysis dose could be obtained using two sessions per week (2 HD/week) if renal clearance was equal to or greater than 2.5ml/min. Starting in 2006, we decided to establish a progressively increasing dialysis regimen at the start of renal replacement therapy, evaluating the possibility of starting with 2 HD/week when renal clearance of urea was equal to or greater than 2.5ml/min.
In this study, we present our experience from the first 5 years of application of this progressively increasing haemodialysis regimen and its repercussions on the maintenance of residual renal function.
MATERIAL AND METHOD
Our criteria for starting haemodialysis treatment in patients with stage 5 chronic kidney disease is a glomerular filtration rate <6ml/min in patients with mild symptoms, or higher levels in patients with symptoms of uraemia or with heart failure that cannot be controlled using conservative treatment. When a patient is admitted to the haemodialysis unit, we perform a study of residual renal function, determining glomerular filtration rate as the mean of urea and creatinine clearance rates. We calculate residual renal function using 24-hour diuresis, as recommended by the 2006 KDOQI guidelines (Guideline 6)18 and by other authors.11-12,16,19-22 Clearance is calculated based on urea and creatinine concentrations in urine samples collected 24 hours prior to the start of the first dialysis session each week and in a blood sample taken immediately before the start of dialysis. The results from the first analysis, usually performed during the first week of dialysis treatment, are considered as the baseline values for glomerular filtration rate.
All patients are dialysed with a high-permeability biocompatible membrane and ultrapure dialysate. The duration of each dialysis session is initially set at 3.30 or 4 hours per session based on whether the patient’s dry weight is greater than or less than 60kg. If urea clearance as measured during the first determination of glomerular filtration rate is equal to or greater than 2.5ml/min, treatment is started with 2 HD/week (Monday and Friday or Tuesday and Saturday) if the patient’s clinical situation allows, based on the criteria of the attending physician.
Residual renal function is analysed every two months until diuresis falls below 100ml/day. After this moment, residual renal function is considered to be null. The prescription of 2 HD/week is maintained until renal clearance of urea falls below 2.5ml/min, or when clinical symptoms and/or alterations in laboratory results appear that would recommend increasing the frequency of dialysis. As a general rule, patients that receive two dialysis sessions per week receive a daily dose of 80mg furosemide (oral) on days in which they do not receive dialysis treatment.
The dialysis dosage is determined for each haemodialysis session using normalised urea clearance (Kt/V) provided by the monitor. Every two months, we also determine Kt/V using the second-generation simplified monocompartmental Daugirdas equation (Kt/V dialysis). The total urea Kt/V (Kt/V total) is calculated by adding the dialysis Kt/V to a fraction corresponding to the urine elimination of urea based on the method described by Gotch.8 Weekly Kt/V is the product of total Kt/V for each session multiplied by the number of sessions per week. Urea production is calculated using the Depner formula23 and normalised protein catabolic rate (PCR-n) is calculated using the Borah formula as modified by Sargent.24
In our study, we included all patients that started treatment with periodic haemodialysis in our haemodialysis unit between 1 January 2006 and 30 September 2010, and that remained on dialysis for more than three months. Follow-up with our study patients ended on 31 December 2010 (end-point for the study) or when haemodialysis treatment was interrupted due to kidney transplant, recovered renal function, transferral to peritoneal dialysis, or death. We considered that the start of dialysis was programmed when patients entered into dialysis with a definitive vascular access.
The rate of decrease in glomerular filtration rate (ml/min/month) and 24-hour diuresis (ml/month) was established using the following formula: in patients that started with 2 HD/week, we calculated the difference between baseline glomerular filtration rate and the glomerular filtration rate obtained at the last measurement before switching to the 3 HD/week regimen, or the glomerular filtration rate at the last measurement prior to ending haemodialysis treatment due to one of the aforementioned causes, or the glomerular filtration rate measured in the patient at the end of the study if the patient continued on the 2 HD/week regimen; the difference was divided by the number of months during which the patient was on the 2 dialysis sessions per week regimen. In patients that started with 3 HD/week, we calculated the difference between baseline glomerular filtration rate and the last glomerular filtration rate measured prior to considering residual renal filtration to have disappeared (diuresis <100ml/day), or the glomerular filtration rate prior to the end of haemodialysis treatment, or the glomerular filtration rate measured prior to the end of the study if the patient continued to have residual renal function at this moment. This difference was divided by the number of months during the period analysed. This same procedure was used to calculate the decrease in diuresis.
In order to evaluate the influence of residual renal function upon variables with clinical relevance, we examined a cross-sectional sample of patients taken in January 2010 in which we examined the relationship of glomerular filtration rate with several clinical and laboratory parameters. Blood pressure was the mean of all pre-dialysis values during the haemodialysis sessions attended during this month. The same procedure was used to determine inter-dialytic weight gain, which was also assessed as a mean value over one month. In order to evaluate the distribution of body water compartments, all patients in the dialysis unit underwent a post-dialysis bioelectrical impedance analysis using a monofrequency bioelectrical impedance vector analysis monitor (EFG ElectroFluidGraph® analyser, Akern SRL, Florence, Italy).
Statistical analysis
Results are expressed as mean ± standard deviation (SD). All data analysed conformed to a normal distribution (Kolmogorov-Smirnov test), and subsequently we used only parametric tests. We compared means using Student’s t-test. For qualitative variables, we used chi-square tests with Yates correction. The correlations between variables were analysed using linear regression models. Survival was calculated using a Kaplan-Meier analysis. P <.05 values were considered to be statistically significant.
RESULTS
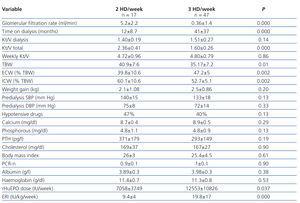
Between 1 January 2006 and 30 September 2010, 95 patients were incorporated into the chronic haemodialysis programme at our hospital. This was the first start on renal replacement therapy for 77 patients, and the other 18 were patients with kidney transplants who had developed chronic graft dysfunction and had to return to haemodialysis. In 41 patients (43%), renal replacement therapy was started at 2 HD/week in light of a renal clearance of urea equal to or greater than 2.5ml/min and an adequate clinical situation according to the doctor in charge. In 54 patients (57%), the initial HD regimen was 3 HD/week; in 29 of these patients, baseline renal clearance of urea was equal to or greater than 2.5ml/min, but the attending physician saw the need for 3 HD/week due to clinical complications such as heart failure, uncontrolled hypertension, or volume overload, necessitating a greater frequency of dialysis sessions. Table 1 shows the characteristics of the study patients at the start of treatment. Patients on the 2 HD/week regimen had a lower mean Charlson index (age-comorbidity), with no differences as compared to the 3 HD/week group in terms of age, sex, programmed start to dialysis, or type of nephropathy. The 2 HD/week regimen was used less frequently in patients that had previously received a kidney transplant: of the 17 patients that had previously received a transplant, only three started on the 2 HD/week regimen.
Of the 41 patients who started on the 2 HD/week regimen, 10 received transplants while on this treatment, one patient was transferred to peritoneal dialysis, 6 recovered renal function and were able to abandon dialysis treatment, 15 were switched to the 3 HD/week regimen, and 9 continued on the 2 HD/week regimen at the end of the study. Of the 15 patients who were transferred to the 3 HD/week regimen, 4 received transplants, 3 died, and the remaining 8 were still on haemodialysis at the end of the study. The mean time that patients remained on the 2 HD/week regimen until the end of the follow-up period was 11.1±7.2 months (range: 2-25 months).
Of the 54 patients that started on the 3 HD/week regimen, 16 received transplants, one patient was transferred to peritoneal dialysis, 3 were switched to the 2 HD/week regimen due to sustained renal clearance of urea equal to or greater than 2.5ml/min and adequate control of the clinical situation of heart failure that had been the cause for placing the patients in the 3 HD/week group, 14 died during the follow-up period, and the remaining 20 patients were still on the 3 HD/week regimen at the end of the study. Of the 3 patients that were switched to the 2 HD/week regimen, two recovered residual renal function to a sufficient degree that they were able to abandon haemodialysis treatment, and the other patient was still receiving two HD sessions per week at the end of the study. Table 2 summarises the global evolution of the two patient groups.
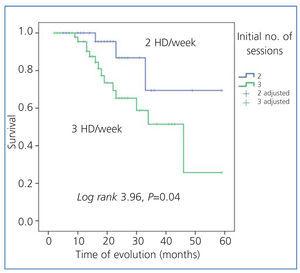
The Kaplan-Meier analysis demonstrated that patients starting on the 2 HD/week regimen had a greater survival rate; log-rank: 3.964; P=.04 (Figure 1).
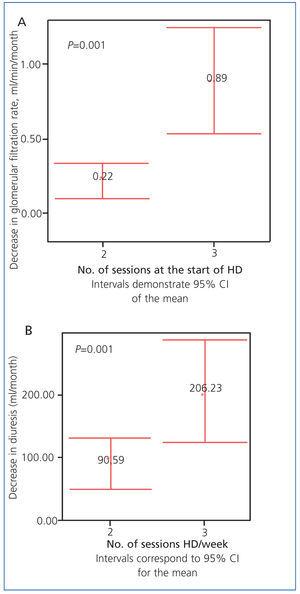
The loss of glomerular filtration rate and 24-hour diuresis was less severe in patients on the 2 HD/week regimen: 0.22±0.36ml/min/month vs. 0.89±1.26ml/min/month for glomerular filtration rate (P=.001) (Figure 2 A), and 90.59±132ml/month vs. 206.23±286ml/month for 24-hour diuresis (P=.001) (Figure 2 B).
In January 2010, a total of 64 patients were being treated in the haemodialysis unit: 17 were on the 2 HD/week regimen and 47 were on the 3 HD/week regimen. Table 3 shows the results from the cross-sectional study. Total weekly Kt/V was similar between the two groups. Total body water was greater in the group of patients on 2 HD/week, but with a greater proportion of intracellular water and a lower proportion of extracellular water.
We did not observe statistically significant differences between the two groups in terms of inter-dialytic weight, pre-dialysis systolic or diastolic blood pressure, percentage of patients on hypotensive drugs, or parameters for mineral metabolism and nutrition. The mean concentration of haemoglobin was also similar between the two groups, but the dose of erythropoietin and the mean erythropoietin resistance index value were both lower in the group of patients on 2 HD/week.
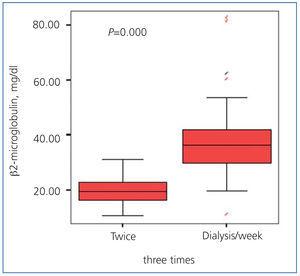
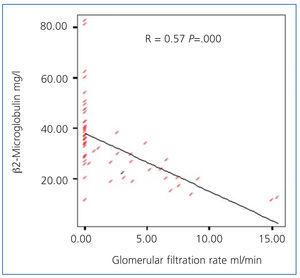
Serum concentrations of β2-microglobulin were significantly lower in the 2 HD/week group (19.7±5 vs. 38.2±13; P=.000) (Figure 3), with a statistically significant inverse relationship between serum concentrations of β2-microglobulin and glomerular filtration rate (Figure 4).
DISCUSSION
Not only is residual renal function a potent predictor for the survival of patients on peritoneal dialysis,3,25 but also for those treated with haemodialysis.22,26-29 In addition to allowing for increased fluid intake, retaining residual renal function also provides benefits for the elimination of medium and large molecules,9-11,16 inflammation,30 correction of anaemia while using lower doses of erythropoietin,27 decreased concentrations of hepcidin,13 nutritional state,27,30-32 quality of life,33 and control of hypertension.34 For these reasons, maintaining residual renal function constitutes a relevant objective for the treatment of patients on periodic haemodialysis, and should be integrated into the process for optimising this treatment.9,12,16,17,35-40
Due to causes that are still not entirely understood, the loss in residual renal function is faster in patients treated with haemodialysis than in those receiving peritoneal dialysis.41-44 Volume depletion45 and episodes of hypotension associated with intermittent dialysis techniques are the primary reasons used to explain this phenomenon.15,44 The factors that have been associated with improved preservation of residual renal function in patients on haemodialysis are control of arterial hypertension,46 absence of obesity,47 the use of ultrapure dialysate,48 and the use of biocompatible membranes,20,49 although this last aspect has not been confirmed in other studies.50 The preservation of residual renal function has not been shown to be influenced by improved control of alterations to mineral metabolism,51 the use of convective techniques,9 or the use of angiotensin-converting enzyme inhibitors.42
The criteria we use to start chronic dialysis treatment plans are similar to those recommended by the Spanish Society of Nephrology, with dialysis commencing when glomerular filtration rates fall below 6ml/min, or at higher glomerular filtration rates in cases of elderly patients, patients with a greater comorbidity, and those with symptoms of uraemia.52 Our criteria for glomerular filtration rates are also similar to those used for the majority of patients starting dialysis that are registered by the European Dialysis and Transplant Association.53 Starting in 2006, we decided to establish a progressively increasing dialysis regimen at the start of renal replacement therapy, commencing with two sessions of dialysis per week if renal clearance of urea in 24-hour urine samples was equal to or greater than 2.5ml/min. The K/DOQI guidelines from 20066 do not recommend an initial dialysis regimen of 2 sessions per week, unless residual urea clearance is greater than 3ml/min/1.73m2. The European Haemodialysis Guidelines suggest a regimen of three sessions per week with a total duration of at least 12 hours per week, except for patients who retain a significant residual renal function, although this range is not established.5 We used the residual urea clearance value of 2.5ml/min as a cut-off value based on the original criteria established by Gotch in 1985.7
Managing extracellular water volume is a factor of special importance for patients on dialysis, with contrasting clinical impacts. While the contraction of extracellular volume accelerates the loss of residual renal function, expansion can also have undesirable effects on arterial hypertension and left ventricular hypertrophy.45 The objective would be to achieve an equilibrium of extracellular volume that would allow for preserving residual renal function but without provoking the negative effects of volume overload.54 In our experience, patients that started haemodialysis with a progressively increasing prescription remained on the 2 HD/week regimen for a mean 11 months, with no relevant complications produced. In the prevalent population analysed, we observed no differences between patients on the two different treatment regimens in terms of weight gain between dialysis sessions, the ability to control blood pressure, or the need for hypotensive drugs. Total body water was greater in the group of patients on 2 HD/week, but with a better distribution between intra and extracellular water components. We also observed a lower concentration of β2-microglobulin and reduced needs for erythropoietin in patients on 2 HD/week.
Preservation of residual renal function is one of the objectives of this progressively increasing prescription. We observed that the monthly decrease in glomerular filtration rate and 24-hour diuresis was less severe in patients on 2 HD/week. In an analysis prior to our own35 and in the study by Lin et al.55 carried out in a prevalent population on haemodialysis, patients treated with 2 HD/week had not only a better residual renal function, but also a less severe decrease in residual renal function in the following six months. In the study by Lin et al., patients treated with two weekly sessions had lower inter-dialytic weight gains, lower blood pressure values, a lower incidence of episodes of hypotension during haemodialysis sessions, a lower incidence of vascular access thromboses, and a lower rate of hospitalisations due to infection.55
In patients treated with peritoneal dialysis, studies have shown that survival and quality of life are closely correlated with residual renal function, not with peritoneal clearance rates.4 In patients treated with haemodialysis, studies have also observed lower survival rates and more frequent hospitalisations in patients who had lost residual renal function.26-29 In anuric patients, mortality rates were higher in those receiving lower doses of dialysis; however, in patients with residual diuresis, the impact of the dialytic dosage on mortality was much less severe.
In our study, we observed that the survival of patients receiving a progressively increasing dialysis dosage was greater, and although this may be due to the fact that these patients had a lower Charlson comorbidity index, it may also be due to the fact that they retained residual renal function for a longer period, with the aforementioned clinical advantages this entails.
As a rule, we administer diuretic medications to patients receiving progressively increasing dialysis doses on days in which they are not administered a dialysis session. Although a wide variation exists in the use of diuretics in patients on haemodialysis,56 the DOPPS study showed that the probability of maintaining residual renal function after one year of haemodialysis treatment was twice as high in patients treated with diuretics.56
Finally, we did not observe a significant correlation between a programmed start to dialysis and the prescription of 2 HD/week, but we must keep in mind that excessive ultrafiltration and the use of nephrotoxic drugs that are required in certain clinical situations, such as heart failure and intervening processes, contribute to the loss of residual renal function at the start of dialysis treatment.
To conclude, the maintenance of residual renal function must be a primary objective in patients starting treatment with haemodialysis, since this provides major benefits in terms of patient survival. In a selected population, dialysis can be commenced under a progressively increasing regimen, starting with 2 HD/week. In our experience, this is a safe prescription that probably contributes to preserving residual renal function.
Conflicts of interest
The authors declare that they have no potential conflicts of interest related to the contents of this article.
Table 1. Data from the start of dialysis
Table 2. Evolution of patients according to initial haemodialysis regimen
Table 3. Relationship between the frequency of dialysis sessions and clinical/laboratory parameters. Cross-sectional sample of prevalent patients
Figure 1. Actuarial survival in both patient groups
Figure 2 A. Decrease in glomerular filtration rate in both patient groups (ml/min/month)
Figure 2 B. Decrease in 24-hour diuresis in both patient groups (ml/month)
Figure 3. Levels of ß2-microglobulin in both patient groups
Figure 4. Relationship between residual glomerular filtration rate and ß2-microglobulin concentration