La cistatina C es un marcador de función renal y predictor de morbi-mortalidad cardiovascular. En la población general, la cistatina C está condicionada por diversos factores independientes de la función renal, pero es poco conocido qué factores se relacionan con esta proteína en fases avanzadas de la enfermedad renal crónica (ERC). Pacientes y métodos: se estudian 52 pacientes no diabéticos (38 hombres, edad media 49 años) en diferentes estadíos de ERC (22 en estadío 3, 25 en estadío 4 y 5 en estadío 5), en los que se determinaron los niveles de cistatina C, filtrado glomerular estimado (MDRD), estado inflamatorio (PCR, IL-6 y fibrinógeno), estrés oxidativo [anticuerpos anti-LDL oxidada, actividad y concentración de paraoxonasa-1 (PON-1)], masa ventricular izquierda (ecocardiograma) y otros factores de riesgo cardiovascular. Resultados: los niveles medios de cistatina C fueron de 2.35±0.9 mg/L. La cistatina C se correlacionó con los niveles séricos de creatinina, filtrado glomerular estimado, niveles de PTH y negativamente con los anticuerpos anti-LDL oxidada. Por el contrario, no se encontró ninguna relación entre esta proteína y los marcadores de inflamación, la actividad y concentración de PON, los niveles de colesterol y sus fracciones, triglicéridos, proteinuria, masa ventricular izquierda ni parámetros demográficos como edad, índice de masa corporal (IMC) o tensión arterial. En un análisis de regresión múltiple, después de ajustar por los niveles de PTH y anticuerpos anti-LDL oxidada, sólo el filtrado glomerular estimado se relacionó independientemente con los niveles de cistatina C (β=-0.500, p=0.001). Conclusión: en pacientes no diabéticos con ERC prediálisis, los niveles de cistatina C están estrechamente relacionados con el grado de disfunción renal. El estado inflamatorio, el estrés oxidativo, la masa cardíaca y otros factores de riesgo cardiovascular no son determinantes de los niveles de cistatina C en fases avanzadas de la ERC. Palabras clave: Cistatina C, inflamación, estrés oxidativo, paraoxonasa-1, factores de riesgo, enfermedad renal crónica.
Cystatin C is a marker of renal function and a major cardiovascular risk factor. In the general population, cystatin C appears to be influenced by factors other than renal function alone. However, information for serum cystatin C levels in chronic kidney disease (CKD) is lacking. Methods: We studied 52 nondiabetic patients (38 men, mean age 49 years) with CKD stage 3 (22), 4 (25) or 5 (5) who had measurements of serum cystatin C levels, estimated glomerular filtration rate (MDRD), inflammatory (C-reactive protein, interleukin-6 and fibrinogen), and oxidative markers (anti-oxidized LDL antibodies, serum paraoxonase-1 activity and concentration), left ventricular mass index by echocardiography and other cardiovascular risk factors. Results: Mean cystatin C levels were 2.35 ± 0.9 mg/l. Cystatin C was positively correlated with creatinine serum levels, estimated glomerular filtration rate, PTH levels and negatively with anti-oxidized LDL antibodies. On the other hand, cystatin C was not related to inflammatory markers, serum paraoxonase-1 activity and concentration, proteinuria, HDL or LDL cholesterol, serum triglycerides, left ventricular mass index or demographic factors such as age, body mass index and blood pressure. After adjustment for PTH levels and anti- oxidized LDL antibodies, only estimated glomerular filtration rate was independently related serum cystatin C levels (β = -0.500, p = 0.001). Conclusion: In nondiabetic patients with CKD, cystatin C is closely related to the degree of renal dysfunction. In contrast, inflammatory state, oxidative stress, left ventricular mass index and other cardiovascular risk factors are not related to cystatin C levels in this population.
INTRODUCTION
Cystatin C is a non-glycosylated, low molecular weight protein that is produced by nucleated cells at a constant rate.
It is filtered through the glomerulus and reabsorbed and degraded at the tubular level, without being reabsorbed into the plasma and without undergoing a tubular secretion process.1 This protein is considered to be a better renal function marker than serum creatinine, particularly in the elderly and in patients with moderate kidney dysfunction.2,3 Epidemiological studies have shown, based on equations derived from creatinine, that cystatin C is better than creatinine or glomerular filtrate as a predictive factor for cardiovascular morbidity and mortality,4-7 especially in elderly patients6,8 and in the general population with no known CKD.9 There has also been recent evidence that cystatin C is associated to global and cardiovascular mortality in nondiabetic patients with stage 3-4 CKD.10 Although it was initially believed that this protein was independent from extra-renal factors,2,11,12 in recent years it has been shown that cystatin C levels may be conditioned by diverse clinical and demographic factors. In this way, age, sex, body type, lipoprotein anomalies, presence of metabolic syndrome, inflammation, arterial hypertension and other factors have been linked in the general population to serum cystatin C levels.13-18 On the contrary, it is not well known what extrarenal factors are related to levels of this protein in pre-dialysis patients with CKD.
The aim of our study was the evaluation of the relationship existing between cystatin C levels and various cardiovascular risk factors, the inflammatory state and oxidative stress in pre-dialysis patients with CKD
MATERIAL AND METHODS
Patients
Prospective transversal study including 52 non-diabetic patients (38 men, 14 women) ranging in age from 30 to 60 years, and with serum creatinine levels between 2 and 8mg/dl. Patients were chosen from those receiving outpatient services at the Nephrology Department at the Joan XXIII University Hospital in Tarragona.
Methodology
For all patients, we proceeded to:
1. Determine the demographic, clinical and therapeutic variables, including BMI and blood pressure, which was measured with an automatic oscillometric monitor (OMRON 705 CP, Healthcare GMBH, Hamburg, Germany) on the seated patient following five minutes of repose, according to the recommendations of the European Societies of Hypertension and Cardiology.19
2. Determine conventional analysis factors, including glycaemia, total cholesterol, high density cholesterol (HDL), low density cholesterol (LDL), triglycerides, albumin, haemoglobin and fibrinogen.
3. Determine the urinary excretion of proteins in urine over 24 hours using the turbidimetric method (Modular DP Hitachi, Roche), with a chemical reagent for precipitation.
4. Determine the oxidative state markers. Paraoxonase: the calculation for serum PON-1 activity is determined by the hydrolysed paraoxonase value at 410mm and 37ºC.20 The reagent contained 1 mM paraoxonase and 1mM CaCl2 in 0.05M glycine buffer solution (pH: 10.5) and the calculation was made in an Ilab 1800 automatic analyser (Instrumentation Laboratory, Milan, Italy) with security measures for manipulating stocks of paraoxonase solution (extraction chamber and protective gloves and mask for the operator). Paraoxonase activity was expressed in IU/l. Serum PON-1 concentration was determined using the ELISA technique.21 Antibodies to oxidized LDL: the detection of antibodies against oxidized LDL was carried out using the ELISA technique and the commercial kit IMTEC-ox-LDL-Antibodies (Immunodiagnostika GmbH) with an intra-essay variation coefficient of 4.6% and inter-assay coefficient of 5.8%.
5. Determine the renal function parameters: serum creatinine levels (modified colourimetric Jaffé method) and estimated glomerular filtration rate calculated using the abbreviated formula (four variables) derived from the serum creatinine values (MDRD).22
6. Determine the serum cystatin C levels in serum that had previously been frozen to -70ºC after thawing using the particle-enhanced immunonephelometry method (N Latex Cystatin C, BN Dade Behring) in a nephelometer (BNII, Dade Behring).23 Reference values ranged from 0.53 to 0.95mg/l. The trial sensitivity was 0.05mg/l; the intra-and inter-assay variation coefficients were under 3.1 and 3.5%, respectively.
7. Determine inflammatory markers:high sensitivity interleukin-6 and high-sensitivity C-reactive protein. We used the kit Human IL-6 Quantikine HS High Sensitivity, R&D Systems. The method sensitivity was 0.016pg/ml and the intra- and inter-assay coefficients were less than 7.8 and 9.6% respectively. High-sensitivity C-reactive protein (hsCRP) was determined in serum samples using the immunonephelometry method (N High Sensitivity CRP) in a nephelometer (BNII, Dade Behring). The sensitivity was 0.175mg/l. The intra- and inter-assay variation coefficients were less than 4.44 and 5.7%, respectively.
8. Standard echocardiographic evaluation in 2D and M mode guided by 2D, using Diasonic 700 or 800 Vingmed Sound device (Horten, Norway) with a 3.5 MHz transductor in parasternal longitudinal and transverse views and subcostal apical 2-chamber and 4-chamber views. According to recommendations by the American Society of Echocardiography,24 we determined the telesystolic and telediastolic diameter of the left ventricle, the thickness of the interventricular septum (IVS) and the thickness of the posterior ventricular wall. The left ventricular mass (LV mass) was calculated using the following formula25: MVI(g) = 0.8 x 1.04 [(DTDVI + SIV + PPVI)3– DTDVI3) + 0.6. The LV mass index was calculated by dividing the LV26 mass by body height and expressed in g/m.2.7
Statistical analysis
Statistical analysis was carried out using the SPSS v11.5 program. The values are expressed as the mean ± standard deviation for variables with a normal distribution.
The hsCRP and IL-6 levels did not present a normal distribution, and were therefore transformed in logarithms for use in the statistical analysis.
The single-variable relationship between cystatin C and the different parameters is determined by using Pearson’s and/or the Spearman correlation coefficient. Multiple linear regression was applied to analyse the relationship between variables. The confidence interval was 95% and differences were considered to be statistically significant at p < 0.05.
RESULTS
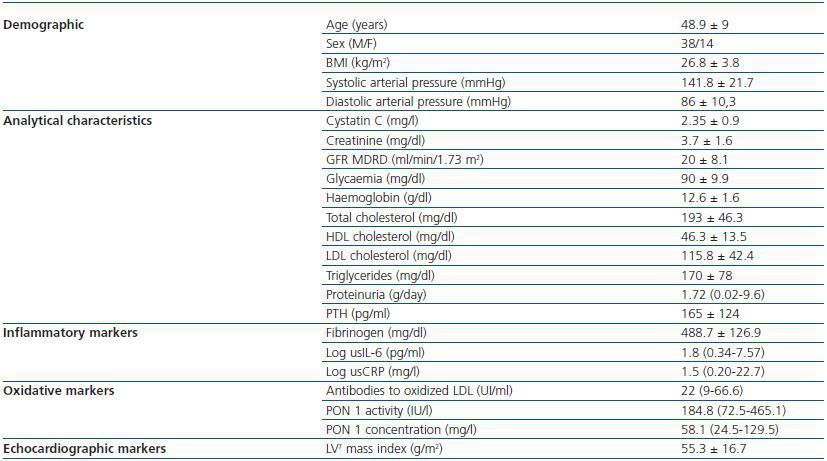
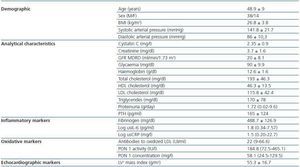
1. Patient characteristics: the demographic, clinical and analytical characteristics are shown in Table 1. All patients had hypertension; 49 were taking antihypertension drugs and 40 were being treated with angiotensin converting enzyme inhibitors and/or angiotensin II receptor antagonists. Sixteen patients were being treated with statin drugs. Twenty-two patients had stage 3 CKD, 25 had stage 4 and five had stage 5. None of the stage-5 patients was on dialysis. The most prevalent cause of CKD was glomerularbased nephropathy (18 patients), followed by nephropathy of tubulo-interstitial origin and polycystic disease, (15 patients), vascular origin (9 patients) and unknown in 10 patients.
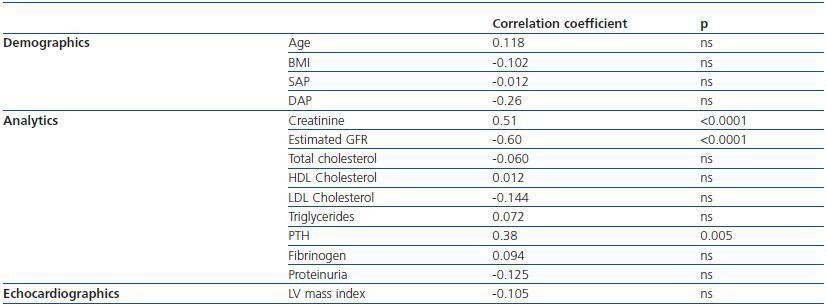
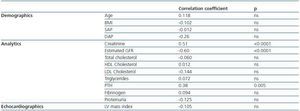
2. Cystatin C, renal function and cardiovascular risk factors: cystatin C levels were 2.35 ± 0.9mg/l; no significant differences between men and women were observed. Cystatin C was significantly related to the creatinine, the MDRD and PTH levels. We did not observe any correlation between this protein and age, BMI, blood pressure, proteinuria, total and partial cholesterol, triglycerides, or with the LV mass index (table 2).
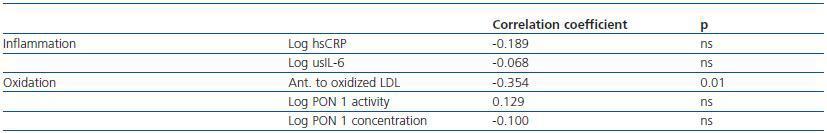
3. Cystatin C, inflammation, and oxidative state: cystatin C levels are negatively correlated with antibodies to oxidized LDL, while no correlations were found between PON activity and concentration or between any of the analysed inflammation markers (table 2). No correlation was observed between HDL and LDL cholesterol levels and the inflammation and oxidation parameters.
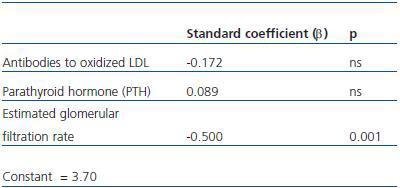
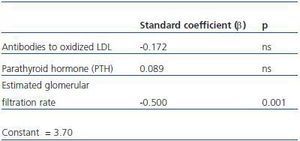
4. Factors independent from cystatin C levels: in a multiple regression analysis, after having adjusted for PTH and antibodies to oxidized LDL, the estimated glomerular filtration rate was independently related to cystatin C levels (table 3).
DISCUSSION
Our results show that in middle-aged non-diabetic patients with pre-dialysis CKD, cystatin C levels are closely related to renal function parameters, and that estimated glomerular filtration rate is the most important and independent predictive factor from the levels of that protein. The relationship between cystatin C levels and PTH was dependent on the renal function, since in the multiple regression analysis the relationship disappeared after adjusting for estimated glomerular filtration rate. On the contrary, diverse cardiovascular risk factors such as age, blood pressure, body mass index, total and partial cholesterol levels, triglyceride level and inflammatory status – all of which have been linked to cystatin C in the general population - showed no relationship in our study. Nor were oxidative state and cardiac mass shown to be linked to this protein. Therefore, in advanced CKD cystatin C levels would be strictly dependant on renal function, and the other extra-renal factors that have traditionally been described in the general population would have a decreasing effect on this protein.
Cystatin C and cardiovascular risk factors
It is well known that cystatin C levels are higher than that estimated glomerular filtration rate derived from serum creatinine as a predictive factor for cardiovascular morbidity and mortality.4-7 This fact is even more noticeable in elderly patients6,8 and in patients with estimated glomerular filtration rate higher than 60ml/min.9 Cystatin C’s superiority over creatinine as a cardiovascular risk factor in patients known to have CKD has been studied less, as has this protein’s dependence on various cardiovascular risk factors. In a recent study10 of non-diabetic patients with stage 3 or 4 CKD which analysed the influence of cystatin C as a global and cardiovascular risk factor, this protein was associated with a higher risk of mortality, as has been similarly observed with serum creatinine or with glomerular filtration rate calculated using iodothalamate. In this study, C cystatin was correlated with BMI, proteinuria, systolic arterial pressure, and inversely correlated with HDL cholesterol levels. However, these correlations were not adjusted for the degree of renal failure, and therefore we cannot exclude a possible influence, per se, of renal failure in some of these correlations.
In our study, we found no association between cystatin C and BMI, systolic arterial pressure, HDL cholesterol levels or triglyceride levels, which are all factors that have been related to this protein in the general population.13,14 The lack of correlation between cystatin C and BMI could probably have been influenced by the different representation of both sexes among the patients included in our study.
Cystatin C and inflammatory status
In the general population, particularly in elderly patients, cystatin C has been correlated with various inflammatory markers.27,28 In our study we found no relationship between cystatin C and CRP, IL-6, or any other component of inflammatory response, such as albumin or fibrinogen. This relationship is dependent on renal function29 in some studies, and it has been suggested that cystatin C, as the most sensitive renal function marker, would be better to reflect the well-known association between the inflammatory state that has been described in patients with subclinical renal dysfunction29 and the characteristic inflammatory state of CKD30 and of patients on dialysis.31
We found no relationship between cystatin C and CRP, IL-6 or any other element of the inflammatory response such as albumin or fibrinogen.
These results are similar to those shown by Menon et al.10 in the above mentioned study of patients found to have CKD. These authors also found no relationship between cystatin C levels and CRP and the association between cystatin C and cardiovascular mortality was independent from the CRP.
The discrepancy observed in various studies between the association of CRP and the severity of renal dysfunction10,32,33 could be due to the fact that the mechanisms in CKD that are responsible for inflammation are not well-defined, and to the presence of various co-morbidities in CKD that also contribute to inflammation in these patients.32 Inflammation markers are predictors of cardiovascular events and mortality in patients on pre-dialysis and dialysis.31,34 Nevertheless, the question has been raised of whether inflammation has causal relevance in the morbidity and mortality of this population. This association could be the result of an inverted causality by which the CKD would cause inflammation and, by means of other mechanisms independent from the inflammation, would also cause an increased risk of cardiovascular events. All of this could suggest that the impact of inflammation on cardiovascular risk in CKD, especially for pre-dialysis CKD patients, could be more modest.32
Cystatin C and oxidative status
Various studies have demonstrated that with CKD there is an increase in the oxidative state characterised by an increase in production of products derived from lipid peroxidation and a decrease in anti-oxidant capacity.35-37 In our study we use two markers to evaluate oxidative status: the antibodies to oxidized LDL and PON. Antibodies to oxidized LDL are atherogenic modulators that have been related to the severity of atherosclerosis and cardiovascular disease,38,39 and some studies have shown that uraemic patients have higher levels of these antibodies than the general population does.40,41
PON is an esterase/lactonase associated with high-density lipoprotein and which circulates in plasma. It is speculated that it degrades active oxidized lipids, acting like an antioxidant system.42 The levels of this enzyme are lower in patients with CKD43-45 and they are a predictive factor that is independent from cardiovascular mortality in this population.46
The relationship between cystatin C and the two oxidative status markers evaluated in our study had not been previously analysed. Our results show that cystatin C is not related with PON activity and concentration, while it is inversely correlated with the level of antibodies to oxidized LDL. Nevertheless, this correlation was dependent upon renal function, since it disappeared when we adjusted for the estimated glomerular filtration rate. In a certain way, these results corroborate the lack of correlation between the severity of the renal failure and the various oxidation markers33 in CKD patients, although they contradict the close relationship shown by other studies between lipid oxidation markers and various antioxidant systems and the degree of renal failure.41
The controversy that exists between the degree of renal dysfunction and the oxidative state could partly be explained by the scarce number of patients in some studies, the presence of other pro-oxidant factors independent from glomerular filtration in CKD patients, and the complexity of evaluation oxidation in those patients. Many of the markers that are used to evaluate the oxidative state are eliminated by the kidney by tubular transport and metabolism, and not by glomerular filtration.33
Cystatin C and cardiac mass
In patients with essential arterial hypertension, cystatin C is an early marker for lesions on target organs related with hypertension, such as the left ventricular mass, the carotid artery intima-media thickness and microalbuminuria.47
In a study previously carried out by our group on hypertensive patients with normal renal function, cystatin C, unlike creatinine and estimated glomerular filtration rate, was independently related the left ventricular mass index, which suggests that this protein could be an early marker of cardiac hypertrophy in the hypertensive population.48 In the Heart and Soul49 study of patients with coronary disease with clinical cardiac failure, cystatin C levels were related to the presence of left ventricular hypertrophy and diastolic dysfunction, and this relationship was closer than that observed with the estimated glomerular filtration rate. We have not found any previous references that evaluate the association between cystatin C and cardiac mass in CKD patients. In our study we could not show any relationship between these two parameters, which was surprising, given that throughout the course of CKD there is a precocious development of left ventricular hypertrophy, and its presence increases as the renal function level decreases.50 In our study, the lack of correlation between cystatin C and cardiac mass could be due to the scarce number of patients and the presence of diverse interrelated factors, including high blood pressure and its treatment. All of the patients were hypertensive: most were receiving treatment, 70% were being treated with renin-angiotensin system blockers, and the degree of control over blood pressure was not equal. All of these factors may have altered the relationship between cystatin c and cardiac mass in some way.
Limitations of the study
The low number of patients included in the study may have influenced the results that were obtained, particularly in the lack of association observed between cystatin C levels and different cardiovascular risk factors, including inflammatory status and oxidative stress.
In conclusion, in advanced CKD cystatin C levels are closely related to the parameters of renal function. On the contrary, inflammatory status, oxidative stress, cardiac mass and other cardiovascular risk factors do not determine the serum levels of this protein.
Table 1. Patient Demographic characteristics
Table 2. Cystatin C, renal function and cardiovascular risk factors
Table 3. Cystatin C, inflammation, and oxidative status
Table 4. Multiple logistic regression analysis with cystatin C as an independent variable