This work presents an update on the management of iron deficiency in patients with chronic kidney disease (CKD), either with or without anaemia. A review is made of the recommendations of the guidelines for the treatment of iron deficiency in CKD. It also presents new studies on iron deficiency in patients with CKD, as well as new findings about iron therapy and its impact on clinical outcomes.
Anaemia is a common complication of CRF, and is associated with a decrease in the quality of life of the patients, as well as an increase in morbidity and mortality. Iron deficiency (absolute or functional) is common in non-dialysis chronic kidney disease patients, and may cause anaemia or a low response to erythropoiesis-stimulating agents. For this reason, the clinical guidelines for the treatment of the anaemia in Nephrology indicate the correction of the deficiency in the presence of anaemia. Iron replacement therapy is indicated in patients with CKD and anaemia (Hb < 12 g/dl) in accordance with the guidelines. There is no unanimity in the indication of iron replacement therapy in patients with Hb > 12 g/dl, regardless of whether they have an absolute or functional iron deficiency.
Intravenous iron replacement therapy is safe, more efficient and rapid than oral therapy for achieving an increase haemoglobin lels and reducing the dose of erythropoiesis-stimulating agents. For the administration of intravenous iron in non-dialysis chronic renal failure patients a strategy of high doses and low frequency would be preferred on being more convenient for the patient, preserves better the venous capital, and is safe and cost-effective. Iron plays an essential role in energy metabolism and other body functions beyond the synthesis of haemoglobin, for which the iron deficiency, even in the absence of anaemia, could have harmful effects in patients with CKD. The correction of the iron deficiency, in the absence of anaemia is associated with functional improvement in patients with heart failure, and in muscle function or fatigue in patients without CKD.
Despite the evidence of benefits in the correction of iron deficiency in patients with CKD, more studies are required to evaluate the impact of the correction of the iron deficiency in the absence of anaemia on morbidity and mortality, quality of life and physical capacity, as well as the long-term effect of oral and intravenous iron replacement therapy in this population.
Este trabajo realiza una actualización sobre el manejo del déficit de hierro en pacientes con enfermedad renal crónica (ERC), ya sea con o sin anemia. Se revisan las recomendaciones de las guías para el tratamiento de la ferropenia en la ERC. Además, muestra los nuevos estudios sobre ferroterapia en pacientes con ERC, así como los nuevos conocimientos sobre el déficit férrico y su impacto en los resultados clínicos.
La anemia es una complicación frecuente de la ERC y se asocia con una disminución en la calidad de vida de los pacientes, así como un aumento de la morbimortalidad. El déficit de hierro (absoluto o funcional) es frecuente en pacientes con ERC que no reciben diálisis, y puede causar anemia e hiporrespuesta a los agentes estimuladores de la eritropoyesis. Por ello las guías clínicas para el tratamiento de la anemia en Nefrología aconsejan la corrección del déficit de hierro en presencia de anemia. La ferroterapia está indicada en pacientes con ERC y anemia (Hb < 12 g/dl) de acuerdo con las guías no existiendo unanimidad en la indicación de ferroterapia en pacientes con Hb > 12 independientemente de que presenten déficit férrico absoluto o funcional.
La ferroterapia intravenosa es segura, más eficaz y rápida que la oral para conseguir aumentar los niveles de hemoglobina, reducir las dosis de agentes estimulantes de la eritropoyesis. Ante la administración de hierro intravenoso en los pacientes con ERC no en diálisis se preferirá una estrategia de altas dosis y baja frecuencia por ser más conveniente para el paciente, preservar mejor el árbol venoso, ser segura y coste-efectiva. El hierro juega un papel esencial en el metabolismo energético y otras funciones del organismo más allá de la síntesis de la hemoglobina, por lo que el déficit de hierro, aun en ausencia de anemia, podría tener un efecto deletéreo en los pacientes con ERC. La corrección de déficit de hierro en ausencia de anemia se asocia con mejoría funcional en pacientes con insuficiencia cardíaca, y de función muscular o de la fatiga en pacientes sin ERC.
A pesar de las evidencias del beneficio en la corrección de déficit de hierro en pacientes con ERC se requieren mas estudios para evaluar el impacto de la corrección del déficit de hierro en ausencia de anemia sobre la morbimortalidad, calidad de vida y capacidad física, así como el efecto a largo plazo de la ferroterapia oral e intravenosa en esta población.
Anemia is a frequent complication of chronic kidney disease (CKD) and is associated with a decrease in the quality of life of patients, as well as an increase in morbidity and mortality and CKD progression.1,2 Although it has been classically described that the main cause of anemia in CKD is the inadequate production of endogenous erythropoietin, in recent years other contributing factors have been recognized, such as a decreased erythropoietic response of the bone marrow due to uremic toxins and inflammatory state, iron deficiency and decreased iron availability for erythropoiesis due to increased hepcidin levels, shortened red blood cell survival or vitamin deficiencies (vitamin B12 or folic acid), among others.3
Iron deficiency (absolute or functional) is common in CKD patients who do not receive dialysis (non-D CKD)4–7 and can cause anemia and hyporesponsiveness to erythropoiesis-stimulating agents (ESAs). Furthermore, it has recently been observed that iron deficiency per se in this population has been associated with a higher risk of adverse outcomes.7–9 Iron administration can increase hemoglobin levels (Hb) in patients with CKD and anemia, and even in some patients with non dialysis CKD, allows to achieve target levels of Hb without ESA.10 If the patient receives ESA, adequate iron parameters must also be ensured before and during treatment, in order to achieve an adequate response and reduce the doses of ESA. However, although the risk-benefit balance is favorable, it should not be forgotten that iron therapy presents some adverse effects and potential risks, the evidence of which will be the subject of the present discussion.
The clinical guidelines for the treatment of anemia in Nephrology advise correction of iron deficiency in the presence of anemia.11–13 However, in other diseases, such as heart failure and in other situations, a clinical benefit of iron deficiency correction has been demonstrated, regardless of the presence or absence of anemia,14 so correction of iron deficiency per se in CKD should be an area of future research in Nephrology.
Iron deficiency in CKD: definitionClassically, the assessment of the iron status in the body in CKD patients has been based mainly on 2° parameters: ferritin levels and the transferrin saturation index (TSAT). However, these values, although widely available, are easily influenced by different factors. This limitation could be improved by using other markers (less accessible and more expensive), such as the percentage of hypochromic red blood cells (provided the sample is processed within 6 h from its extraction), the reticulocyte Hb content, or hepcidin, 15–18 although only the first 2 are recommended as alternatives in the different current clinical guidelines. For this reason, and given the lack of reliability of new markers, we must continue to use the classic markers.
There is consensus on the definition of iron deficiency and the different subtypes that compose it in CKD15,16:
- a
Absolute iron deficiency: situation depletion of iron stores, defined as a serum ferritin concentration < 100 ng/ml and TSAT < 20%.15–17
- b
Functional iron deficiency: defined as an TSAT < 20%and normal/high ferritin levels. In functional iron deficiency, iron stores are normal or high, but iron cannot be adequately mobilized from the reticulo-endothelial system. The causes of functional iron deficiency are treatment with ESA (the iron needs by the bone marrow exceed the ability to mobilize iron from the stores) and inflammation, mainly. In this case, the indication for iron therapy is controversial in terms of the target levels of ferritin and TSAT to achieve in order to obtain a clinical benefit for the patient.15,16
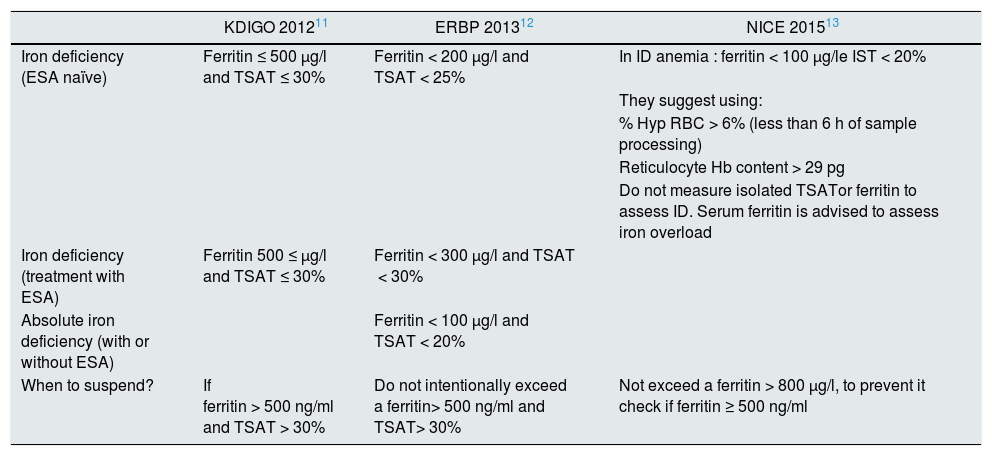
There is unanimity in the clinical guidelines for the indication of iron therapy in patients with CKD, anemia and iron deficiency if there is an absolute iron deficiency (ferritin < 100 ng/ml and TSAT < 20%), but there is no unanimity in other situations, it is especially in relation to the administration of iron in patients with functional iron deficiency.11,19 All of them coincide in correcting iron deficiency with the aim of improving quality of life, avoiding transfusions and delaying the need or reducing ESA doses in anemic patients with CKD and iron deficiency. Table 1 shows the indications for iron therapy according to the different guidelines. European guidelines (ERBP) adopt a more conservative approach in anemic patients with non-dialysis CKD, indicating it to patients without ESA if TSAT < 25% and ferritin < 200 ng/dl if an increase in Hb concentration is desired without start ESAs or if the patient is undergoing ESA treatment, iron therapy would be indicated when the TSAT is< 30% and ferritin < 300 ng/dl if an increase in Hb levels or a reduction in the ESA dose is desired.9 However, the KDIGO guidelines established with an evidence 2C that iron therapy can be initiated in patients with IST < 30% and ferritin < 500 ng/dl, whether or not they receive ESA.8
Indications for iron therapy according to the different clinical guidelines.
| KDIGO 201211 | ERBP 201312 | NICE 201513 | |
|---|---|---|---|
| Iron deficiency (ESA naïve) | Ferritin ≤ 500 μg/l and TSAT ≤ 30% | Ferritin < 200 μg/l and TSAT < 25% | In ID anemia : ferritin < 100 μg/le IST < 20% |
| They suggest using: | |||
| % Hyp RBC > 6% (less than 6 h of sample processing) | |||
| Reticulocyte Hb content > 29 pg | |||
| Do not measure isolated TSATor ferritin to assess ID. Serum ferritin is advised to assess iron overload | |||
| Iron deficiency (treatment with ESA) | Ferritin 500 ≤ μg/l and TSAT ≤ 30% | Ferritin < 300 μg/l and TSAT < 30% | |
| Absolute iron deficiency (with or without ESA) | Ferritin < 100 μg/l and TSAT < 20% | ||
| When to suspend? | If ferritin > 500 ng/ml and TSAT > 30% | Do not intentionally exceed a ferritin> 500 ng/ml and TSAT> 30% | Not exceed a ferritin > 800 μg/l, to prevent it check if ferritin ≥ 500 ng/ml |
ESA: erythropoiesis stimulating agents ; ID: iron deficiency; Hb: hemoglobin; Hyp RBC: hypochromic red blood cells; TSAT: transferrin saturation index.
However, the results of the FIND-CKD studies10 in patients with non-D CKD or the PIVOTAL study in hemodialysis patients20 may modify these objectives in the next guidelines.
European guidelines also state that treat or not to CKD patients, Hb > 12 g/dl and iron absolute deficit is an open question,12 given the absence of evidence of the risk-benefit profile.
Is there an Hb threshold from which to start iron therapy in an anemic patient with CKD and iron deficiency?The recommendation of clinical guidelines on the administration of iron do agree in patients with CKD anemic (Hb < 12 g/dl) with TSAT < 20% and ferritin < 100 ng/ml. No recommendations in anemic patients with non dialysis CKD and functional iron deficiency, given the absence of evidence.11
It is not appropriate to extrapolate the threshold Hb applied for starting ESA prescription (Hb < 10 g/dl), which are lower, based on the results of clinical trials with these agents.11–13 Iron, unlike ESAs, is a necessary factor for an efficient erythropoiesis, but not a growth factor, so there is no risk of overshooting or exceeding the Hb level above the target limit, since the red blood cell production will be regulated by endogenous EPO and if ESA is used, its dose can be reduced, which would improve safety with the latter and reduce costs. On the other hand, we already know from the post hoc analysis of the CHOIR and TREAT studies that the cardiovascular events (CV) of the group with a target Hb >13 g/dl are more associated with the high doses of ESA used to achieve this Hb level, than the Hb value finally reached.21,22 In this regard, patients with CKD and spontaneous Hb > 13 g/dl have no more events than those with lower levels of Hb.23
Is there a route of choice for the administration of iron therapy in CKD?The different clinical guidelines advocate first the administration of iron to replenish the deposits and only when this has been achieved and the anemia persists they suggest starting treatment with ESA.11–13 Although the KDIGO guidelines seem to favor intravenous (IV) iron administration in patients with non-D CKD, they also consider oral iron therapy (PO) as an alternative for 1–3 months in these patients.12
On the other hand, the European recommendations (ERBP) advise that in patients with non-D CKD and mild-moderate anemia, oral iron therapy would be the first-line treatment for a minimum of 3 months.11 IV iron therapy would be indicated if:
-
The objectives of iron parameters are not reached with oral iron therapy for 3 months or when there is intolerance or malabsorption of oral iron.
-
Patients with severe anemia and iron deficiency in whom a rapid Hb response is required.
-
Patients with chronic inflammatory processes showing functional iron deficiency (TSAT < 20% with high-normal ferritin), especially if they require ESA.
The NICE guidelines also advocate the administration of oral iron in patients with non-D CKD, although they comment that some patients will require IV iron. It should be noted that this guide indicates that when IV iron therapy is prescribed in CKD patients who are not on hemodialysis, a high-dose, low-frequency strategy is recommended as the treatment of choice,13 contrary to the strategy suggested in patients on hemodialysis.
The guidelines also recommend avoiding tissue iron accumulation, so they advise that, during treatment with iron, the limits of TSAT of 30% and a ferritin of 500 ng/ml should not be exceeded, both in patients with non-D CKD, and in CKD-5D.11
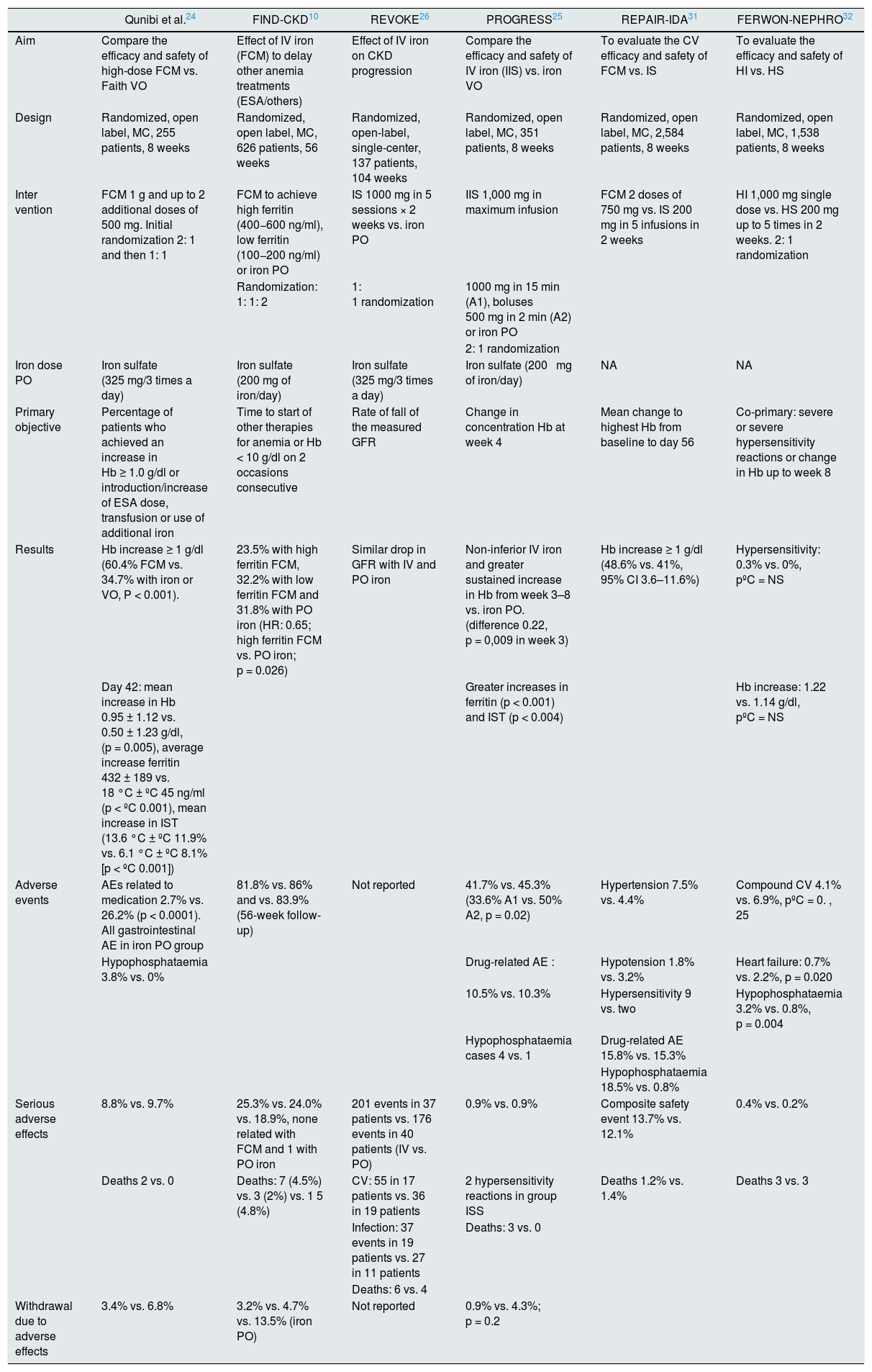
Oral iron therapy vs. intravenous in Non-D CKD: evidence from clinical trialsSeveral studies and meta-analysis have compared the efficacy and safety of IV iron vs. oral iron in patients with non-D CKD, and the most relevant ones will be mentioned here. Table 2 shows the most important randomized IV iron studies in patients with non-D CKD.
Most important randomized studies of IV iron in patients with non D CKD.
| Qunibi et al.24 | FIND-CKD10 | REVOKE26 | PROGRESS25 | REPAIR-IDA31 | FERWON-NEPHRO32 | |
|---|---|---|---|---|---|---|
| Aim | Compare the efficacy and safety of high-dose FCM vs. Faith VO | Effect of IV iron (FCM) to delay other anemia treatments (ESA/others) | Effect of IV iron on CKD progression | Compare the efficacy and safety of IV iron (IIS) vs. iron VO | To evaluate the CV efficacy and safety of FCM vs. IS | To evaluate the efficacy and safety of HI vs. HS |
| Design | Randomized, open label, MC, 255 patients, 8 weeks | Randomized, open label, MC, 626 patients, 56 weeks | Randomized, open-label, single-center, 137 patients, 104 weeks | Randomized, open label, MC, 351 patients, 8 weeks | Randomized, open label, MC, 2,584 patients, 8 weeks | Randomized, open label, MC, 1,538 patients, 8 weeks |
| Inter vention | FCM 1 g and up to 2 additional doses of 500 mg. Initial randomization 2: 1 and then 1: 1 | FCM to achieve high ferritin (400−600 ng/ml), low ferritin (100−200 ng/ml) or iron PO | IS 1000 mg in 5 sessions × 2 weeks vs. iron PO | IIS 1,000 mg in maximum infusion | FCM 2 doses of 750 mg vs. IS 200 mg in 5 infusions in 2 weeks | HI 1,000 mg single dose vs. HS 200 mg up to 5 times in 2 weeks. 2: 1 randomization |
| Randomization: 1: 1: 2 | 1: 1 randomization | 1000 mg in 15 min (A1), boluses 500 mg in 2 min (A2) or iron PO | ||||
| 2: 1 randomization | ||||||
| Iron dose PO | Iron sulfate (325 mg/3 times a day) | Iron sulfate (200 mg of iron/day) | Iron sulfate (325 mg/3 times a day) | Iron sulfate (200 mg of iron/day) | NA | NA |
| Primary objective | Percentage of patients who achieved an increase in Hb ≥ 1.0 g/dl or introduction/increase of ESA dose, transfusion or use of additional iron | Time to start of other therapies for anemia or Hb < 10 g/dl on 2 occasions consecutive | Rate of fall of the measured GFR | Change in concentration Hb at week 4 | Mean change to highest Hb from baseline to day 56 | Co-primary: severe or severe hypersensitivity reactions or change in Hb up to week 8 |
| Results | Hb increase ≥ 1 g/dl (60.4% FCM vs. 34.7% with iron or VO, P < 0.001). | 23.5% with high ferritin FCM, 32.2% with low ferritin FCM and 31.8% with PO iron (HR: 0.65; high ferritin FCM vs. PO iron; p = 0.026) | Similar drop in GFR with IV and PO iron | Non-inferior IV iron and greater sustained increase in Hb from week 3–8 vs. iron PO. (difference 0.22, p = 0,009 in week 3) | Hb increase ≥ 1 g/dl (48.6% vs. 41%, 95% CI 3.6–11.6%) | Hypersensitivity: 0.3% vs. 0%, pºC = NS |
| Day 42: mean increase in Hb 0.95 ± 1.12 vs. 0.50 ± 1.23 g/dl, (p = 0.005), average increase ferritin 432 ± 189 vs. 18 °C ± ºC 45 ng/ml (p < ºC 0.001), mean increase in IST (13.6 °C ± ºC 11.9% vs. 6.1 °C ± ºC 8.1% [p < ºC 0.001]) | Greater increases in ferritin (p < 0.001) and IST (p < 0.004) | Hb increase: 1.22 vs. 1.14 g/dl, pºC = NS | ||||
| Adverse events | AEs related to medication 2.7% vs. 26.2% (p < 0.0001). All gastrointestinal AE in iron PO group | 81.8% vs. 86% and vs. 83.9% (56-week follow-up) | Not reported | 41.7% vs. 45.3% (33.6% A1 vs. 50% A2, p = 0.02) | Hypertension 7.5% vs. 4.4% | Compound CV 4.1% vs. 6.9%, pºC = 0. , 25 |
| Hypophosphataemia 3.8% vs. 0% | Drug-related AE : | Hypotension 1.8% vs. 3.2% | Heart failure: 0.7% vs. 2.2%, p = 0.020 | |||
| 10.5% vs. 10.3% | Hypersensitivity 9 vs. two | Hypophosphataemia 3.2% vs. 0.8%, p = 0.004 | ||||
| Hypophosphataemia cases 4 vs. 1 | Drug-related AE 15.8% vs. 15.3% | |||||
| Hypophosphataemia 18.5% vs. 0.8% | ||||||
| Serious adverse effects | 8.8% vs. 9.7% | 25.3% vs. 24.0% vs. 18.9%, none related with FCM and 1 with PO iron | 201 events in 37 patients vs. 176 events in 40 patients (IV vs. PO) | 0.9% vs. 0.9% | Composite safety event 13.7% vs. 12.1% | 0.4% vs. 0.2% |
| Deaths 2 vs. 0 | Deaths: 7 (4.5%) vs. 3 (2%) vs. 1 5 (4.8%) | CV: 55 in 17 patients vs. 36 in 19 patients | 2 hypersensitivity reactions in group ISS | Deaths 1.2% vs. 1.4% | Deaths 3 vs. 3 | |
| Infection: 37 events in 19 patients vs. 27 in 11 patients | Deaths: 3 vs. 0 | |||||
| Deaths: 6 vs. 4 | ||||||
| Withdrawal due to adverse effects | 3.4% vs. 6.8% | 3.2% vs. 4.7% vs. 13.5% (iron PO) | Not reported | 0.9% vs. 4.3%; p = 0.2 |
CV: cardiovascular; AE: adverse events; FCM: ferric carboxymaltose; GFR: glomerular filtration tare; Hb: hemoglobin; IIS: iron isomaltoside ; IS: iron sucrose; HR: hazard ratio; IV: intravenous; MC: multicenter; PO: oral route.
Qunibi et al. compared treatment with ferric carboxymaltose (FCM) 1 g vs. oral iron (65 mg/d). After 8 weeks of treatment, the increase in Hb levels was higher with FCM (1.31 g/dl vs. 0.83 g/dl, p < 0.01) and a higher percentage of patients than receiving FCM had an increase in Hb ≥ 1 g/dl compared to oral iron therapy (60.4% vs 34.7% of patients), regardless of whether or not they were receiving ESA treatment. Treatment with FCM alone was as effective as combined treatment with PO iron and ESA, and combined treatment with FCM and ESA was superior to treatment with PO iron and ESA.24 Similar results were reported in the PROGRESS study with Iron isomaltoside (IIS).25
The FIND-CKD study is the largest global multicenter study with long follow-up, which compared IV and PO iron treatment in patients with non-D CKD and anemia, not treated with ESA. The study included 626 patients who were randomized to receive FCM 1 g and then every 4 weeks, if necessary, to achieve ferritin levels between 400−600 ng/ml, or FCM 200 mg and then every 4 weeks if it was necessary to maintain ferritin levels between 100−200 ng/ml or iron PO (ferrous sulfate at a dose of 200 mg/day of elemental iron). The primary endpoint was time to initiate an alternative treatment for anemia or occurrence of a trigger of Hb, as defined 2 consecutive values Hb < 10 g/dl or after week 8 without an increase ≥ 0.5 g/dl between consecutive values. 76% of the patients maintained Hb ≥ 10 g/dl or required additional treatment for anemia when treated with FCM in order to achieve high levels of serum ferritin (hazard ratio [HR] 0.65, IC 95%: 0.44 to 0.95, p = 0.026 for FCM and high ferritin vs. oral iron). Likewise, the percentage of patients who achieved an increase in Hb ≥ 1 g/dl was higher in the high FCM ferritin group (56.9% vs. 34.2% low FCM-ferritin group and 32.1% oral iron, p < 0.01). The high FCM-ferritin group had higher Hb and ferritin levels than the other 2 groups throughout the 52 weeks of study. Regarding safety, there were no differences between the groups in terms of adverse events or serious adverse effects between the different groups, but there was a higher dropout rate in the PO iron group, especially due to gastrointestinal adverse events.10
The REVOKE study was a single-center, randomized clinical trial that compared the effect of IV iron sucrose (IS) vs. oral iron therapy in patients with non-D CKD on renal function as the primary event.26 The study was stopped prematurely due to the increased risk of adverse events in the group treated with IV iron, which generated controversy in the nephrology community. However, the number of patients who experienced adverse events was similar in both groups (55 vs. 58%), but when the total number of adverse events was considered, it was higher in the IV iron group: 199 per 100 patient-years vs. 168.4 per 100 patient-years. Serious adverse effects occurred repeatedly in the same patients in the IV iron group (201 events in 37 patients vs 176 events in 40 patients in the PO iron group), but this difference only reached significant differences after adjusting for baseline characteristics . Thus, it is a single-center study in which only 99 patients completed the study. Most adverse events occurred long after the intervention was completed, which ended 8 weeks after randomization, so adverse effects that occurred after 3 months may or may not be related to treatment. The safety signal occurred due to the presence of repeated adverse effects in the same patients and after adjusting. Therefore, and according to the Cochrane criteria (version 5.1.0.) this study has a high risk of bias27 (Table 2).
The meta-analysis by Shepshelovich et al. included 24 trials, 13 involving 2,369 patients with CKD stages 3–5 and 11 that included 818 patients with CKD stage 5D. Patients treated with iron IV were more likely to get an increase in Hb > 1 g/dl (HR 1.61, 95% CI: 1.39–1.87, p < 0.00001) and ferritin levels were significantly higher in all IV iron groups vs. the iron PO group (mean difference between formulations 238.9 ng/ml, 95% CI: 194.3, 283.5, p < 0.00001). Regarding safety, analysis showed comparable rates of adverse and serious adverse events from iron IV and PO (1.06, 95% CI: 0.88–1.28, p = 0.53). However, the follow-up periods were generally limited to 3 months, thus the authors acknowledge that more trials with longer follow-up periods are required to understand the consequences of different treatments on Hb levels and clinical outcomes. The authors concluded that the IV iron treatment is preferred, both for patients with CKD stage 5D, as for those with nonD non D CKD stages 3-5.28 These results have recently been confirmed by the meta-analysis carried out by the Cochrane in which 39 studies (3,852 participants) were examined that compared IV iron supplements versus PO.29
Finally, in a post hoc analysis of the FIND-CKD study, it was described that in those patients randomized to oral iron therapy, at 4 weeks after starting treatment, only 21.6% of anemic patients with non-D CKD and iron deficiency showed an increase in Hb ≥ 1 g/dl. Among those who did not respond at 4 weeks, less than 30% responded at the end of the study (week 52), suggesting that the lack of early response to oral iron treatment could be a reason to consider IV iron therapy,30 which would make it possible to indicate IV iron therapy earlier and correct anemia earlier.
The REPAIR-IDA study analyzed a high-dose, low-frequency strategy with a low-dose, high-frequency strategy. Study31 included 2,584 patients who were randomized to 2doses of FCM 750 mg in 1 week or IS 200 mg administered in up to 5 infusions in 14 days. In the FCM group, a greater number of patients achieved an increase in Hb ≥ 1 g/dl (48.6% vs. 41.0%; 95% CI: 3.6%–11.6%). There were no differences in the composite safety event of cardiovascular death, heart attack and stroke, but there were more episodes of transient hypertension in the FCM group. Therefore, it is concluded that this high-dose, low-frequency strategy is as safe and effective as a multiple-infusion strategy of low-dose ISa.
The FERWON-NEPHRO study was a randomized, open-label, multicenter trial conducted in the USA. Patients were randomized 2: 1 to a single dose of 1,000 mg of IIS 1,000 or IS in doses of 200 mg up to 5 times. In a period of 2 weeks. A total of 1,538 patients were included (mean eGFR 35.5 ml/min/1.73 m2). The safety objective was met based on the absence of significant differences in the incidence of severe or severe hypersensitivity reactions in the IIS and IS groups (0.3% vs. 0%; risk difference: 0.29% [95% CI: –0.19, 0.77; p > 0.05]). The incidence of the composite CV adverse events was significantly lower in the ISS group vs. IS (4.1% vs. 6.9%; p = 0.025, of which heart failure occurred in 0.7% in IIS vs. 2.2% in IS, p = 0.02; hypertension 1, 1% vs. 2%, p = 0.16, and atrial fibrillation 0.3% vs. 1.2%, p = 0.067). Compared with the IS, the IIS produced a higher Hb increase during the first 4 weeks (1.06 vs. 0.91 g/dl, p ≤ 0.021) and the change in Hb at 8 weeks showed no inferiority. The authors concluded that, compared to multiple doses of IS, a single dose of IIS induced a non inferior hematologic response at 8 weeks, comparatively low rates of hypersensitivity reactions, and a significantly lower incidence of the composite CV adverse event.32
Thus, the high-dose, low-frequency strategy recommended by the NICE guidelines13 demonstrates better convenience for the patient with a good efficacy and safety profile.
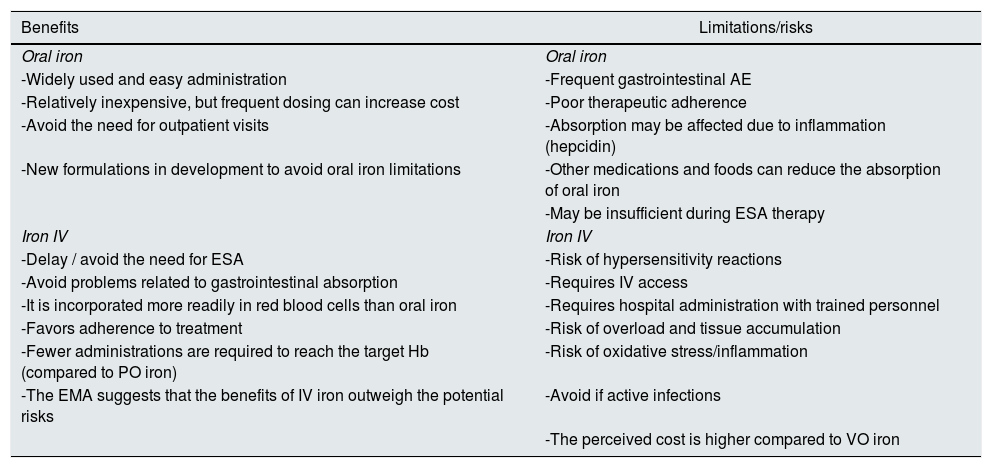
Risks and benefits of different iron formulations in CKDTable 3 shows the benefits as well as the limitations and risks of both PO iron and IV iron. Oral iron supplements can be taken as capsules, tablets, chewable tablets, and in liquid solution. This route of administration is simple, cheaper and preserves the venous capita, which should be considered in these patients given the future need for hemodialysis treatment. In patients with non-D CKD or on peritoneal dialysis in which oral iron is prescribed, the doses recommended in the guidelines in an adult patient will be around 200 mg/day of elemental iron divided into 2–3 doses (better ferrous salts for its better absorption) and preferably on an empty stomach. However, recent studies in non-renal patients seem to show greater efficacy and tolerance of low doses of oral iron, since small increases in iron levels increase hepcidin levels, limiting its intestinal absorption. Recent studies show better absorption of oral iron when administered every other day or in a single daily dose,33,34 which suggests that changing the frequency of administration increases the efficacy of treatment in iron-deficient patients and may improve tolerability. The main problems associated with oral iron treatment in CKD are gastrointestinal intolerance, intestinal absorption problems, or lack of therapeutic compliance associated with its adverse events. Although there are differences in the appearance of side effects, constipation and diarrhea, along with the change in the color of the stool (black) are very common. Nausea and vomiting can occur, and liquid iron formulations can stain teeth. Since non-heme iron absorption is modest, high doses of oral iron can induce oxygen-free radical-mediated toxicity from unabsorbed iron on the intestinal mucosa. On the other hand, oral iron supplements could negatively affect the intestinal microbiota, already altered in CKD.35 This phenomenon has not been sufficiently studied in kidney patients, but it could negatively affect the production of uremic toxins or increase intestinal permeability to the passage of bacterial products into the circulation, aggravating inflammation in these patients,36 as has been shown in other diseases.37
Risks and benefits of iron therapy PO vs IV.
| Benefits | Limitations/risks |
|---|---|
| Oral iron | Oral iron |
| -Widely used and easy administration | -Frequent gastrointestinal AE |
| -Relatively inexpensive, but frequent dosing can increase cost | -Poor therapeutic adherence |
| -Avoid the need for outpatient visits | -Absorption may be affected due to inflammation (hepcidin) |
| -New formulations in development to avoid oral iron limitations | -Other medications and foods can reduce the absorption of oral iron |
| -May be insufficient during ESA therapy | |
| Iron IV | Iron IV |
| -Delay / avoid the need for ESA | -Risk of hypersensitivity reactions |
| -Avoid problems related to gastrointestinal absorption | -Requires IV access |
| -It is incorporated more readily in red blood cells than oral iron | -Requires hospital administration with trained personnel |
| -Favors adherence to treatment | -Risk of overload and tissue accumulation |
| -Fewer administrations are required to reach the target Hb (compared to PO iron) | -Risk of oxidative stress/inflammation |
| -The EMA suggests that the benefits of IV iron outweigh the potential risks | -Avoid if active infections |
| -The perceived cost is higher compared to VO iron |
ESA: erythropoiesis stimulating agents, AE: adverse effects; EMA: European Medicines Agency; IV: intravenous; PO: oral route.
It has been suggested that alternative oral iron compounds might be more effective or better tolerated in this population (sucrosomial iron, ferric citrate, polypeptide heme iron, or ferric maltol, etc.). Of these, ferric citrate has shown repletion of iron stores, partial correction of anemia, and also a reduction in phosphorus, PTH-i and FGF-23.38 Ferric citrate has been approved as a phosphate binder in dialysis patients and has recently also been approved by the Food and Drug Administration for the treatment of iron deficiency anemia in patients with CKD. At the present time, it is not available in Spain (https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205874s013lbl.pdf). However, the evidence in CKD of the other compounds is limited to recommend their use and in some cases they are approved as nutritional supplements and not as drugs, with limitations in terms of evidence of efficacy and safety, in addition to having a higher cost for the patient.39,40
In the administration of IV iron in patients with non-D CKD, the preservation of the venous capital should be considered due to the future need for vascular access for hemodialysis. For this, it is convenient to administer iron coinciding with the performance of analytical controls, thus avoiding more punctures, and the administration of iron at high doses and with less periodicity, as recommended by the NICE guidelines.13 The most important adverse events related to the administration of IV iron compounds are hypersensitivity reactions to it or to any of the excipients. In the aforementioned meta-analysis, a higher risk of arterial hypotension was also reported with IV administration vs. PO.28,29 Likewise, the development of transient hypophosphataemia has been described with IV iron therapy, especially with FCM.41,42 Although it has been described in some studies, and in relation to the administration of IV iron, increased oxidative stress, impaired kidney function, risk of infections or iron accumulation, this has not been confirmed by others. In this sense, the aforementioned meta-analysis did not observe differences in mortality, infection, changes in glomerular filtration rate, or the need for dialysis between IV and oral iron therapy.28,29 More recently, in the PIVOTAL study in hemodialysis patients, those patients randomized to high doses of IV iron did not demonstrate an increased risk of infections.43 These concerns led to a controversial document from the KDIGO in which 4 of these aspects were analyzed and concluded44:
-
Hypersensitivity reactions: The risk of hypersensitivity reactions secondary to IV iron is currently low. However, recent recommendations from the European Medicines Agency (EMA) have sensitized the medical community to these possible negative consequences of serious reactions that occur in circumstances in which there is no adequate organization for such urgent care.
-
Inflammation and oxidative stress: IV iron can cause oxidative damage to the DNA of peripheral blood lymphocytes in hemodialysis patients, protein oxidation and lipid oxidation. Although high doses of IV iron are associated in some studies with a higher mortality rate in the United States dialysis population, it is not clear that iron administration promotes atherosclerosis, arterial remodeling or CV disease, being controversial and inconclusive the results of all studies so far. In fact, at the last EDTA congress a prespecified secondary analysis of the PIVOTAL study was presented, which demonstrated that the proactive iron strategy reduced the risk of type 1 myocardial infarction (of atherosclerotic origin) (European Renal Association-European Dialysis and Transplant Association [ERA-EDTA] 57th Congress. Abstract MO016. Presented June 8, 2020).
-
IV iron and infections: Despite inconclusive results from systematic reviews and meta-analyzes, the benefits and risks of iron therapy should be considered and the administration of IV iron should be avoided in the presence of active infection. However, it must be taken into account that the suspension of iron therapy can lead to an iron deficiency, which in turn induces an increased risk of infection.
-
Iron overload: Pathological iron overload represents a condition of high body iron content associated with signs of organ dysfunction that are presumably caused by excess iron. Although some studies have shown an increase in iron content at the liver level, its clinical relevance is unknown and deposits have not been observed in other territories, such as at the cardiac or pancreatic level.45–47 However, an association between hepatic iron overload and hepatic steatosis has recently been described,48 whose clinical significance is unknown. Despite the lack of evidence, the document recommends avoiding the combination of elevated TSAT and ferritin values.
These conclusions seem to be confirmed in a recent meta-analysis of clinical trials and observational studies in hemodialysis (receiving higher doses of IV iron) that has not found an increased risk of mortality, infections, hospitalization or CV disease in patients who received high doses of IV iron However, this meta-analysis has its limitations, as the follow-up was relatively short and the studies were not designed or powered to analyze directly measurable and clinically relevant outcomes . In observational studies there was a high degree of heterogeneity and inevitably presented a prescription bias.49 On the other hand, a recent review of recommended reading has not shown that a positive iron balance in CKD is associated with clinical toxicity.50 The aforementioned results of the PIVOTAL study also demonstrate the CV and infection risk safety of a proactive IV iron strategy in hemodialysis patients.20,43
Is there evidence of correction of iron deficiency beyond anemia?: heart failure and other situationsImportance of iron deficiency and its correction in heart failureClinical guidelines and consensus documents in patients with heart failure defined iron deficiency in patients with heart failure as ferritin levels < 100 μg/l or 100−300 μg/l associated with TSAT < 20%. According to these criteria, it is estimated that between 30 and 50% of patients with heart failure present an iron deficiency51,52 and the prevalence of iron deficiency increases as the functional class of the New York Heart Association worsens or with increased levels of NT-proBNP.53 Iron deficiency in heart failure worsens functional status,54 quality of life54,55 and exercise capacity, and is associated with a higher risk of morbidity and mortality, which is independent of the presence or absence of anemia.52,56,57 For this reason, iron deficiency is currently considered an important comorbidity in patients with heart failure and constitutes a new therapeutic objective in these patients.51,52
3 large randomized clinical trials have been conducted in patients with heart failure with reduced ejection fraction (HFrEF) and iron deficiency, with a similar design and the same definition of iron deficiency: 2° placebo-controlled studies, the Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure (FAIR-HF) and the Ferric CarboxymaltOse evaluation on performance in patients with IRon deficiency in coMbination with chronic Heart Failure (CONFIRM-HF), and an open study, the Effect of Ferric Carboxymatose on Exercise Capacity in patients with Iron Deficiency and Chronic Heart Failure (EFFECT-HF), with a follow-up of up to 52 weeks.58–61 These studies, which used IV FCM, demonstrated an improvement in the symptoms of heart failure, an improvement in functional class and quality of life, as well as functional capacity. Furthermore, in the CONFIRM study there was a reduction in the risk of hospitalization due to exacerbation of heart failure.60 Likewise, an improvement in renal function was observed with this treatment in a subanalysis of the FAIR-HF study.62 In a small study in patients with heart failure, CKD, and iron deficiency anemia, IV iron therapy was associated with improved myocardial function and cardiac dimensions,63 as in another pilot study.64 The Myocardial-IRON trial has shown changes in cardiac magnetic resonance imaging after the administration of FCM, indicative of myocardial iron repletion in patients with HFrEF.65 A recent meta-analysis on the effect of IV iron therapy in patients with HFrEF and iron deficiency demonstrated a reduction in the combined variable of death from any cause, CV death or hospitalization for heart failure, improvement in functional class, improvement in symptoms, exercise capacity and quality of life measured using different scales.66 Another meta-analysis of the studies with FCM in this population yielded similar clinical results, although the beneficial effect was less in patients with TSAT > 20%.67 On the contrary, the administration of oral iron has not shown benefits in this population.68,69
For all the above, in patients with HFrEF and iron deficiency, the recent guidelines of the European Society of Cardiology (ESC) on heart failure indicate that treatment with IV FCM should be considered in symptomatic patients with heart failure with reduced ejection fraction and iron deficiency in order to improve symptoms, exercise capacity and quality of life (recommendation of class II a and level of evidence A).70 However, the same guideline highlights that no clinical trial had sufficient statistical power to evaluate the effects on hard outcomes (mortality or CV events) or to analyze separately the effects in anemic vs. not anemic. The effect of treating iron deficiency in patients with heart failure with preserved or slightly reduced ejection fraction, and the long-term safety of IV iron in this population are unknown. Currently, several clinical trials are underway that analyze the effect of IV iron administration in patients with heart failure and preserved ejection fraction (FAIR-HFpEF, NCT03074591), in acute heart failure (Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency [AFFIRM-AHF, NCT02937454]), or on morbidity and mortality: Effectiveness of Intravenous Iron Treatment vs. Standard Care in Patients With Heart Failure and Iron Deficiency: A Randomized, Open-label Multicentre Trial (IRONMAN, NCT02642562), Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity & Mortality (FAIR-HF2, NCT03036462), Randomized Placebo-controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency (HEART-FID, NCT03037931), or the Intravenous Iron in Patients with Severe Chronic Heart Failure and Chronic Kidney Disease (NCT00384567), which will definitively answer whether the benefit of iron therapy in patients with heart failure and iron deficiency it also extends to hard outcomes and improves the prognosis in these patients.
There is considerable inter - relationship between heart failure, iron deficiency and renal failure and adding each comorbidity reduces survival of these patients.71 Data from large registries show that CKD is present in 12%–74% of heart failure cases72 and that its prevalence increases with the decrease in renal function.73
We must remember that iron is essential in various cellular processes, such as oxygen transport and storage, as well as in energy metabolism (a component of the mitochondrial electron transport chain).74,75 The heart has the maximum metabolic demands of the body and energy production, largely determined by mitochondrial function, must match energy requirements.75 At the tissue level, the iron content in the myocardium is reduced by 20–30% in patients with severe heart failure.76,77 The activity of iron regulatory elements (IRP1 and IRP2) is decreased in heart failure, associated with a decrease in the expression of the transferrin receptor and the concentration of tissue iron.78 This decrease in myocardial iron is associated with myocardial mitochondrial dysfunction in patients with heart failure.79 In studies with isolated cardiomyocytes or in animal models, iron deficiency at the cellular level is associated with cardiomyocyte dysfunction, which is restored with iron administration.78,80,81 In patients with heart failure and iron deficiency, administration of IV FCM getting repletion of iron myocardial and was associated with remodelling of the left ventricle.64 Thus, in heart failure there is a decrease in the myocardial iron content, which plays a role in the energy generation processes through the mitochondrial respiratory chain, as well as in the release of calcium through the sarcoplasmic reticulum,82 for what its decrease in patients with heart failure could contribute to its pathophysiology. The systemic administration of iron can reverse this situation and improve the symptoms in patients with HFrEF.
Therefore, in patients with heart failure, reduced ejection fraction and CKD, iron deficiency defined as a ferritin < 100 μg/l or ferritin 100−300 μg/l if the TSAT is < 20%, regardless of the presence or absence of anemia, should be corrected with FCM, as indicated in the clinical guidelines for heart failure.70 The anemia group of the SEN published a review recommending the administration of IV FCM in patients with CKD, HFrEF and iron deficiency, even in the absence of anemia, extrapolating the recommendations of the heart failure guidelines.83 In this sense, in the recent PIVOTAL study in hemodialysis patients, a proactive strategy of iron administration vs. a reactive one reduced the risk of hospitalization for heart failure, confirming its clinical benefit also in the dialysis population.20
More recently, it has been reported that iron deficiency is common in patients with acute coronary syndrome and is associated with a worse prognosis, even after adjusting for multiple variables, such as degree of heart failure, myocardial necrosis or anemia.84
Iron deficiency in other situationsBeyond heart failure, there is also evidence that correction of iron deficiency may be associated with clinical improvement in other situations.
Skeletal muscleIron deficiency negatively impacts muscle function, as it plays a crucial role in skeletal muscle function, especially in the context of energy metabolism. Oxidative metabolism cell depends greatly on the availability of iron, which is essential for both the oxygen supply sufficient (myoglobin) and for efficient oxidative phosphorylation.85 In diseases such as heart failure, chronic obsructive lung disease or type 2 diabetes mellitus, a decrease in performance and exercise capacity has been described, in which they seem to coexist with similar histological abnormalities at the muscle level and are the result of mechanisms Parallel molecular pathogens in which iron deficiency seems to play a relevant role.85,86 IV iron administration appears to improve muscle function in iron-deficient patients in some, but not all, studies.86–88 Although myopathy is common and its pathophysiology is more complex in CKD, iron deficiency could contribute to it.
Fatigue/lack of energyIron deficiency has also been associated with fatigue, independently of anemia.74 In the PREFER study premenopausal patients with iron deficiency and fatigue-limit normal Hb (≥115 g/l) were randomized to 1000 mg of FCM or placebo. A 50% reduction in the Piper Fatigue Scale score was observed in 33.3% of the patients treated with FCM vs. 16.4% placebo (p < 0.001). Mental quality of life (SF-12) and cognitive function also improved more with FCM.89
These results have been corroborated in a meta-analysis that demonstrated an improvement in fatigue in subjects with iron deficiency without anemia.90
Improved cognitive and neurological functionIron plays a key role in neurotransmission and brain development and maturation.74 It is also involved in the synthesis and storage of various neurotransmitters and in myelination. In man, iron deficiency anemia has been associated with poorer cognitive and motor functions, but the impact of iron deficiency without anemia is less clear. Studies on iron supplementation in iron-deficient children or adolescent women have not yielded conclusive results on higher functions.91,92
A known effect of iron deficiency on neuronal function is its contribution to restless legs syndrome,74 common in patients with CKD. Animal studies suggest that this is due to alterations in dopamine metabolism due to the lack of available iron92 and a recent Cochrane analysis confirmed its positive effect in these patients.93
Finally, it should be mentioned that iron participates in many other cellular functions, such as DNA replication and repair or the cell cycle, it is present in cytochrome P450 enzymes or the regulation of the immune system,74 among others, so the Correcting its deficit, regardless of the presence or absence of anemia, could have benefits in CKD patients beyond anemia, although studies to confirm this are lacking.
During the article editing process, the AFFIRM-AHF study was published. Multicenter, randomized, double-blind, placebo - controlled study, which included patients hospitalized for acute heart failure with ejection fraction < 50% and iron deficiency (defined as ferritin < 100 μg/l or 100−299 μg/l with a TSAT < 20%). After stabilizing the patient and before hospital discharge, participants were randomly assigned to receive IV FCM or placebo for up to 24 weeks, dosed according to the level of iron deficiency. The primary event was a composite of hospitalization for heart failure or CV death. There were 293 primary events (52.7 per 100 patient-years) in the FCM group and 372 (72.5 per 100 patient-years) in the placebo group (rate ratio [RR] 0.79, CI of the 95%: 0.62–1.01, p = 0.059). The risk of total hospitalizations for CV cause and CV death was reduced by 20% in the FCM group vs. the placebo group (RR 0.80, 95% CI: 0.64–1.0, p = 0.05). There were no differences in CV death among 2 grupos (HR 0.96, CI 95%, from 0.7 to 1.32, p = NS). Total hospitalizations for heart failure were significantly lower in the FCM group (RR 0.740, 95% CI 0.58−0.94, p = 0.013) than in placebo. In addition, benefits were seen in other secondary events (first hospitalization for heart failure or CV death, or days lost due to hospitalization for heart failure and CV death). Serious adverse events occurred in 45% of patients in the FCM group and 51% of patients in the placebo group. The authors concluded that in patients with iron deficiency, left ventricular ejection fraction less than 50%, who had stabilized after an episode of acute heart failure, treatment with ferric carboxymaltose was safe and reduced the risk of hospitalization for heart failure, with no apparent effect on the risk of CV death. Thus, the benefit of IV iron carboxymaltose treatment on “hard” clinical events (mortality and CV events) in patients with heart failure with reduced ejection fraction is beginning to be demonstrated.94
Key points
-
Iron deficiency is common in patients with non-D CKD and is associated with the development of anemia.
-
The iron deficiency can be either absolute (depletion of iron stores with serum ferritin concentration < 100 ng/ml and transferrin saturation index < 20%) or functional (transferrin saturation index < 20% and a normal/high ferritin concentration) and the latter can present with normal or high iron stores, and is related to the difficulty to adequately mobilize iron from the reticulo-endothelial system.
-
The iron therapy is indicated in patients with CKD and anemia (Hb < 12 g/dl) according to guidelines. The guidelines propose the initiation of IV iron therapy or alternatively oral treatment. If at 3 months the oral route is not effective or tolerated, it is advisable to switch to the IV route.
-
IV iron therapy is more effective and faster than oral iron therapy to increase Hb levels, reduce ESA doses and is safe.
-
A High dose and low frequency strategy will be the preferred option for the administration of IV iron in non-D CKD as it is more convenient for the patient, better preserves the venous capital, is safe and cost-effective.95
-
Iron plays an essential role in energy metabolism and other body functions, beyond the synthesis of Hb, so iron deficiency, even in the absence of anemia, could have a deleterious effect on CKD patients.
-
Correction of iron deficiency in the absence of anemia is associated with functional improvement in patients with heart failure and of muscle function or fatigue in non-renal patients.
-
Isolated iron therapy cannot generate overcorrection of Hb levels, although its use in combination with ESAs may be associated with a need to adjust the doses of the latter.
-
Clinical relevance of iron deficiency, regardless of anemia in CKD, evidence from observational studies points to a worse prognosis associated with iron deficiency in this population.
-
Long-term efficacy and safety of oral and IV iron therapy in patients with CKD.
-
Effect of oral iron therapy on the intestinal microbiota, intestinal permeability and systemic inflammation in CKD.
-
More effective dose and administration intervals (daily or every other day) of oral iron therapy in CKD.
-
Controlled studies evaluating the impact of iron deficiency correction in the absence of anemia on morbidity and mortality, quality of life and physical capacity.
The authors have not received support for the submitted work.
AC reports grant and honoraria for lectures and consulting from Vifor Pharma, grant and honoraria for consulting from AB-Biotic, honoraria for lectures and consulting from Astellas, Astra Zeneca and Novo Nordisk; honoraria for consulting from GSK and honoraria for lectures from Amgen and from Bayer, outside the submitted work.
MJP and JLG report honoraria for lectures from Vifor Pharma, outside the submitted work.
PS reports honoraria for lectures from Vifor Pharma, and a grant and participation on an Advisory Board from Astellas, outside the submitted work.
BQ reports honoraria for lectures or support for attending meetings and/or travel from Vifor-Pharma, Astellas, Amgen, Ferrer, Novartis, AstraZeneca, Sandoz, Laboratorios Bial, Esteve, Sanofi-Genzyme, Otsuka; participation on Advisory Boards from AstraZeneca and Laboratorios Bial, outside the submitted work. Currently he is the Secretary of Sociedad Española de Nefrología (S.E.N.).
LMR reports honoraria for lectures from Vifor Pharma and Astellas, and support for attending meetings and/or travel from Baxter.
JP reports participation as Principal Researcher in clinical trials on anaemia conducted by Amgen, Roche, Astellas and GSK, from Amgen, Vifor-Pharma, GSK and Astellas, and honoraria as advisor from Astellas and GSK.
Please cite this article as: Cases A, Puchades MJ, de Sequera P, Quiroga B, Martin-Rodriguez L, Gorriz JL, et al. Ferroterapia en el manejo de la anemia en la enfermedad renal crónica no en diálisis: perspectiva del grupo de anemia de la S.E.N. Nefrologia. 2021;41:123–136.