Ischemia–reperfusion injury causes various severe morphological and functional changes in diabetic patients. To date, numerous antidiabetic and antioxidant agents have been used for treatment of the disease-related changes.
ObjectivesWe aimed to examine effective therapeutic doses or doses of berberine against renal ischemia/reperfusion injury (IRI) in a streptozotocin (STZ)-induced diabetic rat model by histopathological and biochemical analysis.
MethodsThirty male Sprague Dawley rats were treated with STZ injection for the development of diabetes, and divided into the following groups: STZ-induced diabetic group (STZ); IRI-induced diabetic group (STZ+IRI); 50mg/kg berberine (BRB) treated diabetic group after inducing IRI (STZ+IRI+BRB1); 100mg/kg BRB treated diabetic group after IRI (STZ+IRI+BRB2); 150mg/kg BRB treated diabetic group after IRI (STZ+IRI+BRB3). Bilateral renal ischemia model was applied for 45min, then reperfusion was allowed for 14 days in STZ-induced diabetic rats. Renal injury was detected histopathologically. Blood urea nitrogen (BUN), creatinine and lactate dehydrogenase (LDH) levels were measured in serum using the ELISA method. Total antioxidant status (TAS) and total oxidant status (TOS) of renal tissue was studied by spectrophotometric assay. Oxidative stress index (OSI) was calculated as TOS-to-TAS ratio. Tumor necrosis factor alpha (TNF-α), C-reactive protein (CRP), Na+/K+-ATPase (sodium pump), and Ca2+-ATPase (calcium ATPase) enzyme levels were measured in tissues using the ELISA method. Anti-apoptotic Bax and pro-apoptotic Bcl-2 protein levels were detected by Western blot analysis. All data were evaluated statistically.
ResultsThe highest histopathological score was detected in the STZ+IRI group compared to the other group. BRB administration at the doses of 100mg/kg and 150mg/kg markedly improved renal injury. BUN and creatinine levels significantly increased in the STZ+IRI group compared to the STZ group (p<0.001). 100mg/kg and 150mg/kg BRB administration significantly decreased those levels (p<0.01). The highest TOS and the lowest TAS levels were detected in the STZ+IRI group (p<0.001). IRI markedly aggravated inflammation via increasing levels of TNF-α and CRP (<0.001), and caused apoptosis via inducing Bcl-2 protein, and suppressing Bax protein (p<0.001). BRB administration at the doses of 100mg/kg and 150mg/kg showed anti-oxidant, anti-inflammatory and anti-apoptotic effects (p<0.01). The LDH enzyme, was used as a necrosis marker, was higher in the STZ+IRI group than other groups. BRB administration at all of the doses, resulted in the decline of LDH enzyme level (p<0.001). Ca2+-ATPase and Na+/K+-ATPase enzyme activities decreased in the STZ+IRI group compared to the STZ group (p<0.001), while BRB administration at the doses of 100mg/kg and 150mg/kg significantly increased those of enzyme activities, respectively (p<0.05).
ConclusionIschemia with diabetes caused severe histopathological and biochemical damage in renal tissue. The high doses of berberine markedly improved histopathological findings, regulated kidney function via decreasing BUN and creatinine levels, and rearranged intercellular ion concentration via increasing Na+/K+-ATPase and Ca2+− ATPase levels. Berberine showed anti-oxidant, anti-apoptotic, and anti-inflammatory effects. According to these data, we suggest that berberine at the doses of 100 and 150mg may be used as a potential therapeutic agent to prevent renal ischemic injury.
La reperfusión de la isquemia provoca graves cambios morfológicos y funcionales en los pacientes diabéticos. Hasta la fecha numerosos antidiabéticos y agentes antioxidantes han sido utilizados para el tratamiento de los cambios relacionados con la enfermedad.
ObjetivosEl objetivo fue examinar las dosis terapéuticas efectivas o las dosis de berberina (BRB) frente a la insuficiencia renal por isquemia/reperfusión (IRI) en estreptozotocina (STZ) inducida por el modelo de rata por análisis histopatológico y bioquímico.
MétodosTreinta ratas machos Spraque-Dawley fueron tratadas con inyección de STZ para el desarrollo de diabetes, se dividieron en los siguientes grupos: grupo diabético inducido por STZ; grupo diabético inducido por IRI (STZ+IRI); grupo diabético tratado con 50mg/kg de BRB después de la inducción de IRI (STZ+IRI+BRB1); grupo diabético tratado con 100mg/kg de BRB después de IRI (STZ+IRI+BRB2), y grupo diabético tratado con 150mg/kg de BRB después de IRI (STZ+IRI+BRB3). Se aplicó un modelo de isquemia renal bilateral durante 45min, luego se permitió la reperfusión durante 14 días en ratas diabéticas inducidas por STZ. La lesión renal fue detectada histopatológicamente. Los niveles de nitrógeno ureico en sangre (BUN), creatinina y lactato deshidrogenasa (LDH) se midieron en suero por el método ELISA. El estado antioxidante total (TAS) y el estado oxidante total (TOS) del tejido renal se estudiaron mediante un ensayo espectrofotométrico. El índice de estrés oxidativo (OSI) fue calculado como la relación TOS-a-TAS. El factor de necrosis tumoral alfa (TNF-α), proteína C reactiva (CRP), Na+/K+-ATPasa (bomba de sodio) y la Ca2+-ATPasa (calcio ATPasa) de la enzima se midieron los niveles en los tejidos mediante el método ELISA. Los niveles de proteína anti-apoptótica Bax y pro-apoptótica Bcl-2 se detectaron por el análisis de Western blot. Todos los datos fueron evaluados estadísticamente.
ResultadosLa mayor puntuación histopatológica fue detectada en el grupo STZ+IRI en comparación con el otro grupo. La administración de BRB en dosis de 100 y 150mg/kg mejoró notablemente la lesión renal. Los niveles de BUN y creatinina aumentaron significativamente en el grupo STZ+IRI en comparación con el grupo STZ (p<0,001). La administración de 100 y 150mg/kg de BRB disminuyó significativamente esos niveles (p<0,01). Los TOS más altos y los niveles más bajos de TAS se detectaron en el grupo STZ+IRI (p<0,001). El IRI agravó notablemente la inflamación a través del aumento de los niveles de TNF-alfa y de PCR (<0,001), y causó la apoptosis a través de la inducción de la proteína Bcl-2 y la supresión de la proteína Bax (p<0,001). La administración de BRB con las dosis de 100 y 150mg/kg mostró efectos antioxidante, antiinflamatorio y antiapoptóticos (p<0,01). La enzima LDH que se usó como marcador de necrosis fue mayor en el grupo STZ+IRI que en los otros grupos. La administración de BRB en todas las dosis, dio lugar a una disminución del nivel de la enzima LDH (p<0,001). Las actividades de las enzimas Ca2+-ATPasa y Na+/K+-ATPasa disminuyeron en el grupo STZ+IRI en comparación con el grupo STZ (p<0,001, mientras que la administración de BRB en dosis de 100 y 150mg/kg incrementó significativamente las actividades de las enzimas, respectivamente (p<0,05).
ConclusiónLa isquemia con diabetes causó graves daños histopatológicos y bioquímicos en el tejido renal. Las altas dosis de BRB mejoraron notablemente los hallazgos histopatológicos, la función renal regulada a través de la disminución de los niveles de BUN y creatinina, y la concentración de iones intercelulares reordenados mediante el aumento de los niveles de Na+/K+-ATPasa y la Ca2+-ATPasa. La BRB mostró efectos antioxidantes, antiapoptóticos y antiinflamatorios. De acuerdo con estos datos, sugerimos que la BRB en dosis de 100 y 150mg se puede usar como un agente terapéutico potencial para prevenir la lesión isquémica renal.
Renal ischemia/reperfusion injury (IRI) inducing multiple organ dysfunction results in high mortality rates in clinical and experimental studies.1–3 IRI causes various biochemical and morphological alterations including increased intercellular sodium and calcium concentration, intracellular ATP depletion, oxidative stress, tubular and glomerular damage, inflammation, fibrosis, and cell death via apoptosis or necrosis.3–7
Diabetes mellitus (DM), common chronic metabolic disorder, is a serious risk factor for ischemic acute renal failure. IRI accompanying by diabetes adversely affects development of severe diabetic nephropathy.8–10
Berberine (BRB) which is the major active component of Rhizoma Coptidis, has been widely used in Chinese for traditional treatment for diabetes. BRB decreases elevated blood glucose level, and improves insulin sensitivity in diabetic patients and also experimental diabetic animals.11,12 BRB also has an antioxidant activity against oxidative stress.13 Recently, it has been reported that BRB possesses some additional effects such as anti-inflammatory, anti-bacterial, and anti-viral.13–15 On the basis of these features, BRB has been used in several studies, especially ischemia/reperfusion studies as a therapeutic agent.
The aim of the present study was to explore the most effective therapeutic dose of berberine on renal ischemia/reperfusion injury in streptozotocin-induced diabetic rats by using histopathological and biochemical analysis. To our knowledge, this is the first report related with the dose-dependent therapeutic effects of berberine on renal ischemia/reperfusion in STZ-induced diabetic rats.
Materials and methodsDrugs and chemicalsBerberine chloride (BBR) was purchased from Sigma-Aldrich, Germany (B3251). Streptozotocin (STZ; ab142155) was obtained from Abcam, Cambridge, United Kingdom.
For enzyme linked immunosorbent assays (ELISA), tumor necrosis factor alpha (TNF-α, ab100785), C reactive protein (CRP, ab108827), lactate dehydrogenase (LDH, ab102526), and creatinine (ab65340) kits were purchased Abcam, Cambridge, United Kingdom while only blood urea nitrogen (BUN, SL1053Ra) was purchased in SunLong Biotech, Hangzhou, Zhejiang, China).
Primer antibodies (IgG type rabbit polyclonal antibodies) including Anti-β-actin (ab8227), Anti-Bcl-2 (ab196495) and Anti-Bax (ab32503) antibodies for the Western blot analysis were purchased from Abcam, Cambridge, United Kingdom.
Study designAnimalsAnimals were allowed free access to food and water at controlled room temperature (22–25°C) under a 12:12-h day/night cycle for the duration of the study. All procedures were approved by the Animal Care and Use Committee at Bezmialem Vakif University and performed in accordance with institutional guidelines.
Thirty male Spraque Dawley rats (Experimental Animal Research Laboratory at Bezmialem Vakif University, Istanbul, Turkey) were divided into five groups as follows: STZ-induced diabetes group (STZ, n=6); STZ-induced diabetes+renal ischemia/reperfusion group (STZ+IRI, n=6); STZ-induced diabetes+renal ischemia/reperfusion+50mg/kg berberine treatment group (STZ+IRI+BRB1, n=6); STZ-induced diabetes+renal ischemia/reperfusion+100mg/kg berberine treatment group 100mg/kg berberine treatment group (STZ+IRI+BRB2, n=6); STZ-induced diabetes+renal ischemia/reperfusion+150mg/kg berberine treatment group (STZ+IRI+BRB3, n=6).
Diabetes inductionExperimental diabetes mellitus was induced by a single intraperitoneal injection of STZ at the dose of 50mg/kg. It was prepared in 0.1mol/l citrate buffer (pH 4.5), immediately before use. The blood samples at the 24th hour after STZ injection were obtained from the caudal veins. Rats with a glucose concentration higher than 300mg/dL were considered as diabetic.16
Renal ischemia/reperfusion procedureBilateral renal ischemia model was performed on diabetic rats. Animals were anesthetized with intraperitoneal ketamine (4mg/100g) and xylazine (1.5mg/100g) before the onset of the surgery. All animals were shaved and sterilized. Both left and right kidneys were exposed by lateral incisions. Then, left and right renal arteries and veins were isolated. Renal ischemia was achieved by clamping by left and right renal arteries with vascular clips for 45min.17 At the end of this time, vascular clips were removed, and reperfusion was allowed. Finally, incisions were closed with surgical suture. During the surgical procedure, body temperature was monitored using a Nimomed® infrared thermometer.
Berberine administrationBerberine was freshly prepared every day by dissolving in distilled water. The different doses (50mg/kg, 100mg/kg, and 150mg/kg) of berberine were administrated by oral gavage after inducing renal ischemia/reperfusion (IRI). Initial berberine doses were applied at 24th hour after IRI, and daily administrations were made for 14 days. All experimental animals were sacrificed at 24th hour after the all doses were over.
Histological analysisEach kidney sample was divided into two equal pieces. One piece was used for histological analyses, the other was used for biochemical analyses. For histological analysis, tissues were fixed in 10% formalin solution and after dehydration, embedded in paraffin. Five-micrometer (μm) sections obtained from paraffin blocks were stained with Harris Hematoxylin (Bio-optica Catalog no. 05-06004/L, Milano, Italy) and Shandon Eosin Y Alcoholic (Thermo scientific, Catalog no. 6766007, Kalamazoo, MI, USA), Masson Trichrome (Bio-optica Catalog no. 04-010802, Milano, Italy), and Periodic Acid Schiff stains (Beslab BS-0040, Turkey). Hematoxylin & Eosin (H&E) staining technique was used for the detection of pathological alterations including proximal/distal tubular atrophy and necrosis, congestion, and infiltration. Masson's trichrome staining technique was used for detecting, and determining the degree of the fibrosis. Periodic Acid Schiff staining technique was used for detecting of the status of the brush border and glomerular basement membrane.
Scoring system for evaluation of the kidney injuryTotal kidney injury for each sample was investigated by monitoring proximal tubular damage (proximal tubular dilation and atrophy, loss of brush border, proximal tubular necrosis), distal tubular damage (distal tubular dilation/atrophy and distal tubular necrosis), glomerular damage (glomerular basement membrane thickening), interstitial damage (interstitial fibrosis, hemorrhage, and mononuclear cell filtration) and vascular damage (interstitial congestion). All findings were evaluated and scored as follows: 0=absent (<10%), 1=minimal (10–25%), 2=mild (25–40%), 3=moderate (40–55%), 4: severe (>50%) with a maximum score of 40 for each kidney sample.
Sections were scored by two blind observer (Kumas, M and Esrefoglu, M.) using a Nikon Eclipse i5 light microscope with a Nikon DS-Fi1c camera, and Nikon NIS Elements version 4.0 image analysis systems (Nikon Instruments Inc., Tokyo, Japan).
Biochemical analysisOxidative stress parametersOxidative stress parameters including total antioxidant status (TAS) and total oxidant status (TOS) levels were determined by using an automatic biochemical analyzer (c800, Abbott, USA). Tissue TAS level was determined by Erel18,19. After this assay relied on the ability of antioxidants in the sample to inhibit ABTS (2,2′-azino-di-3-ethylbenz-thiazoline sulfonate) from being oxidized into ABTS+ by a peroxidase metmyoglobin. An antioxidant with a known concentration (1.65mmol/l) was used as the standard to calculate antioxidant levels in the samples. The TAS level was expressed as mmol Trolox equivalent/l (mmol Trolox equiv./l). The tissue TOS level was measured by Erel.19,20 TOS method which relied on the oxidation of ferrous ions into ferric ions in the presence of various oxidative species in an acidic medium. Ferric ion concentrations were measured by xylenol orange. The assay was calibrated with a standard hydrogen peroxide solution (39.16μmol/l). Results were expressed as μmol H2O2 equivalent/l (μmol H2O2 equiv./l). The TOS/TAS ratio was defined as oxidative stress index (OSI).
Immunoblot assaysKidney tissues were homogenized in RIPA buffer (50mmol/l Tris–HCl, pH 7.4, 150mmol/l NaCl, 5mmol/l EDTA, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 1% aprotinin, 50mmol/l NaF, 0.1mmol/l Na3VO4), and proteinase inhibitor cocktail (Merck KGaA, Darmstadt, Germany). After centrifugation at 14,000rpm (Beckman Coulter, Krefeld, Germany) for 10min at 4°C, the final supernatant was used as the total protein. Protein concentrations were determined using the Bradford method, then all protein samples were equilibrated at 40mg/ml with loading buffer (4% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.004M Tris–HCl, pH 6.8) and loaded 8% and 12% sodium dodecyl sulfate polyacrylamide gel wells as 30μl, transferred onto a polyvinylidene difluoride membrane (PVDF), and then incubated with primary antibodies including Anti-β Actin Anti-Bax, and Anti-Bcl 2 in a solution containing 5% powdered skim milk and 0.05% Triton X-100/TBS, and washed three times in TBS-T for 10min each. Blots were incubated with a horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG at a concentration of 1μg/ml in 5% powdered skim milk in 0.05% TBS-T. Protein bands were visualized with Pierce ECL Western blotting substrate (Thermo Scientific). Protein bands were visualized using the enhanced chemiluminescence detection system (Vilber Lourmat Fx7, Collégien, France) and analyzed with Image J software.
Enzyme-linked ImmunoSorbent Assay (ELISA)Serum and tissue samples were thawed and ELISA kits were used for the quantitative measurement of Rat TNF alpha, CRP, and BUN. Optical density was read on a standard automated plate reader at 450nm (Perkin Elmer, 1420 Victor 3).
Assays of lactate dehydrogenase (LDH) and creatinineSerum LDH and creatinine levels were detected by colorimetric method using microplate reader (Perkin Elmer, 1420 Victor 3).
Assessment of Na-K-ATPase and Ca-ATPaseNa-K-ATPase and Ca-ATPase levels were measured with ELISA test in kidney tissues. The ELISA assays performed with proteins which isolated from homogenized tissues according to manufacturer's instructions. Results were expressed in nanogram per milliliter of 1mg total protein.
Statistical analysisNormality of the all data was tested with Shapiro–Wilk normality test, because of our sample size of less than 50. Data have a normal distribution (Shapiro–Wilk test, p≥0.05) was analyzed by parametric test ANOVA (post hoc Tukey's HSD) for multiple comparisons, while non-normal distributed data (Shapiro–Wilk test, p<0.05) was analyzed by Kruskal–Wallis test for multiple comparison and Mann–Whitney U test for comparing two groups.
Values are presented as minimum, maximum and median for non-parametric data, while as mean± standard deviation (SD) for parametric data. p values≤0.05 were approved as statistically significant. Statistical analyses were done with SPSS 20.0 (IBM, New York, USA) and MS Office Excel. Graph Pad Prism 6 was used for drawing bar charts.
ResultsWe examined both left and right kidney for histological and biochemical analysis, and compared all results. We did not find any important differences in terms of all evaluated parameters among left and right kidneys. Thus, we preferred to mention only pathological alterations observed in left kidneys.
Assessment of histopathological alterationsThe results about histopathological scores of the all of the groups are presented in Table 1.
Summary statistics and Mann Whitney U test results about histopathological scores in the all groups.
| Left kidney | Pathological alterations | STZ (n=6) | STZ+IRI (n=6) | STZ+IRI+BRB1 (n=6) | STZ+IRI+BRB2 (n=6) | STZ+IRI+BRB3 (n=6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum to maximum (median) | p value | Minimum to maximum (median) | p value | Minimum to maximum (median) | p value | Minimum to maximum (median) | p value | Minimum to maximum (median) | p value | ||
| Proximal tubular damage | Proximal tubular dilation and atrophy | 1–2 (1) | 0.007 (U=2.00) | 2–3 (3) | – | 1–3 (2.50) | >0.05 | 1–2 (1) | 0.004 (U=1.00) | 0–2 (1) | 0.004 (U=1.00) |
| Loss of brush border | 0–1 (1) | 0.003 (U=0.00) | 2–4 (3) | – | 1–2 (1.50) | 0.011 (U=3.00) | 0–1 (0.50) | 0.003 (U=0.00) | 0–1 (0) | 0.003 (U=0.00) | |
| Proximal tubular necrosis | 0–2 (1) | 0.004 (U=0.50) | 2–4 (3.50) | – | 1–3 (2.50) | >0.05 | 1–2 (2) | 0.007 (U=2.00) | 0–2 (1) | 0.004 (U=0.50) | |
| Distal tubular damage | Distal tubular dilation and atrophy | 1–2 (1) | 0.008 (U=2.00) | 2–4 (3.50) | – | 1–4 (2.50) | >0.05 | 1–2 (1.50) | 0.012 (U=3.00) | 1–2 (1) | 0.004 (U=1.00) |
| Distal tubular necrosis | 0–2 (1) | 0.005 (U=1.00) | 2–4 (3) | – | 1–3 (2) | >0.05 | 1–3 (2) | >0.05 | 1–2 (1) | 0.008 (U=2.00) | |
| Glomerular damage | Glomerular basement membrane thickening | 0–1 (0) | 0.027 (U=5.00) | 0–2 (2) | – | 0–2 (1) | >0.05 | 0–1 (0) | 0.014 (U=4.00) | 0–1 (0) | 0.014 (U=4.00) |
| Tubulointerstital damage | Interstitial fibrosis | 0–1 (0.50) | >0.05 | 0–1 (0) | – | 0–2 (0) | >0.05 | 0–1 (0) | >0.05 | 0–3 (0.50) | >0.05 |
| Hemorrhage | 0–3 (1.50) | 0.005 (U=1.00) | 3–4 (4) | – | 0–4 (2) | 0.029 (U=5.00) | 0–1 (1) | 0.003 (U=0.00) | 0–2 (0) | 0.003 (U=0.00) | |
| Mononuclear cell infiltration | 0–2 (1) | 0.008 (U=2.00) | 2–4 (3) | – | 1–3 (2) | 0.008 (U=2.00) | 0–2 (0.50) | 0.006 (U=1.00) | 0–1 (0.50) | 0.003 (U=2.00) | |
| Vascular damage | Glomerulointerstitial congestion | 0–3 (2) | 0.020 (U=4.00) | 2–4 (3) | – | 0–3 (1) | 0.014 (U=3.00) | 0–2 (1) | 0.004 (U=0.50) | 0–1 (1) | 0.003 (U=0.00) |
| Total damage | 4–17 (10) | 0.004 (U=0.00) | 25–28 (27) | – | 10–26 (15.50) | 0.009 (U=2) | 6–11 (10) | 0.004 (U=0.00) | 3–10 (8.50) | 0.004 (U=0.00) | |
The data are expressed median, minimum and maximum using non-parametric Kruskal–Wallis test. p value<0.05 was considered significant. All results belong to STZ+IRI compared to the other groups, and p values were represented in the columns.
The highest proximal tubular damage scores including tubular dilation and atrophy (Fig. 1a), loss of brush border (Fig. 1b), and tubular necrosis (Fig. 1c) were detected in STZ+IRI group. The median scores of each of those of alterations were also statistically higher in STZ+IRI group than STZ group (p=0.007, p=0.003, and p=0.004, respectively). Proximal tubular damage seen in STZ group was severely aggravated after IRI. 50mg dose of BRB administration was not improved proximal tubular dilation and atrophy, and tubular necrosis in STZ+IRI+BRB1 group (p>0.05, Fig. 2a and b), whereas the loss of brush border score was significantly decreased in that group (p=0.011). All of those alterations improved after 100mg and 150mg BRB administration in STZ+IRI+BRB2 (p=0.004, p=0.003, and p=0.007, respectively; Fig. 2c) and STZ+IRI+BRB3 groups (p=0.004, p=0.003, p=0.004, respectively; Fig. 2d).
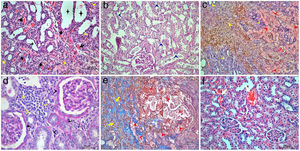
Light microscopic figures of renal cortex in STZ+IRI group. (a) Tubular dilatation, atrophy (asterisks), thyroidization (black arrows) and interstitial mononuclear cell infiltration (yellow arrowheads) are obvious. Hematoxylin & Eosin; 20×. (b) Tubular degeneration (dark blue arrowheads) is obvious. Basement membranes are not clearly seen, brush border loss is prominent. Periodic Acid Schiff; 20×. (c) Tubular necrosis (red arrowheads) and interstitial cell infiltration (yellow arrowheads) are clearly seen. Note that hemosiderin is accumulated within the area. Hematoxylin & Eosin; 10×. (d) Interstitial mononuclear cell infiltration (yellow arrowheads) and glomerular and tubular basement membrane thickening is observed. Periodic Acid Schiff; 40×. (e) Tubular degeneration (red arrowheads), interstitial cell infiltration, and fibrosis (yellow arrows) are seen. Masson's trichrome 20×. (f) Capillary dilatation and congestion are observed. Hematoxylin & Eosin; 20×.
Histological sections of STZ+BRB1 (a and b), STZ+BRB2 (c), and STZ+BRB3 groups (d). Dose-dependent improvement on all of the histological alterations induced by diabetes (STZ) and ischemia reperfusion injury (IRI) is observed. (a) and (c) Hematoxylin & Eosin stain; (b) and (d) Periodic Acid Schiff stain; 20×).
Distal tubular damage including tubular dilation and atrophy (Fig. 1a), and tubular necrosis (Fig. 1c) severely increased in STZ+IRI group compared to STZ group (p=0.008 and p=0.005, respectively). In both STZ+IRI+BRB2 and STZ+IRI+BRB3 groups, distal tubular dilation and atrophy scores markedly decreased when compared to STZ+IRI group (p=0.012 and p=0.004, respectively; Fig. 2c and d). Distal tubular necrosis score reduced in STZ+IRI+BRB3 (p=0.008; Fig. 2c and d), but it was not detected any statistically significant alteration in other berberine administrated groups (p>0.05). Thyroidization which is the similarity of the tubules, accumulating acidophilic and PAS positive material within the lumen, with thyroid follicles was observed in two sections (Fig. 1a). Brown hemosiderin granules accompanied by mononuclear cell infiltration in the necrotic area were also observed (Fig. 1c).
Glomerular basement membrane seemed to be thickened in STZ+IRI group compared to STZ group (p=0.027, Fig. 1d). Berberine administration was effective in normalization of the basement membrane in STZ+IRI+BRB2 and STZ+IRI+BRB3 (p=0.014, for both). Fibrosis and mononuclear cell infiltration related with interstitial damage were observed in STZ+IRI group. When compared to STZ group, mononuclear cell infiltration score was markedly increased in STZ+IRI group (p=0.008, Fig. 1c and d). Berberine administration at all doses significantly improved those scores (p<0.05). Fibrosis was sometimes observed in STZ+IRI group, but it occupied large area in only one sample (Fig. 1c). Berberine administrations had no significant effect on fibrosis.
Congestion and dilatation of the capillaries were even for per sample so were collectively scored (Fig. 1f). The highest median score was detected in STZ+IRI group (p=0.02). All doses of berberine resulted in decrease in congestion scores (p=0.014, p=0.004, and p=0.003, respectively).
The highest total histopathological score was detected in STZ+IRI group (p=0.004). The scores of STZ+IRI+BRB2, and STZ+IRI+BRB3 groups (p=0.004, for both) were lower than that of STZ+IRI group.
Assessment of biochemical alterationsThe results of biochemical parameters of all of the groups are represented in Table 2.
Summary statistics and One-way ANOVA test (post hoc Tukey HSD) results about biochemical analysis in the all groups.
| Biochemical parameters | STZ (n=6) | STZ+IRI (n=6) | STZ+IRI+BRB1 (n=6) | STZ+IRI+BRB2 (n=6) | STZ+IRI+BRB3 (n=6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | p value | Mean±SD | p value | Mean±SD | p value | Mean±SD | p value | Mean±SD | p value | |
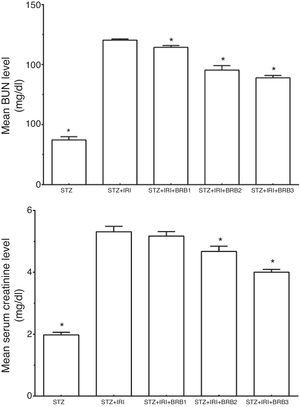
| Blood urea nitrogen (BUN) (mg/dl) | 37.19±2.79 | <0.001 | 120.32±0.98 | – | 114.29±1.55 | 0.002 | 95.37±3.76 | <0.001 | 88.96±1.95 | <0.001 |
| Creatinine (mg/dl) | 1.98±0.08 | <0.001 | 5.31±0.17 | – | 5.17±0.15 | >0.05 | 4.68±0.17 | <0.001 | 4.00±0.09 | <0.001 |
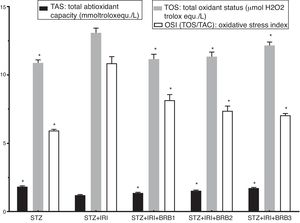
| Total antioxidant capacity (TAS) (mmol Trolox equiv./l) | 1.84±0.06 | <0.001 | 1.21±0.04 | – | 1.36±0.06 | <0.001 | 1.55±0.05 | <0.001 | 1.73±0.05 | <0.001 |
| Total oxidant status (TOS) (μmol H2O2 Trolox equiv./l) | 10.83±0.22 | <0.001 | 13.08±0.35 | – | 11.18±0.32 | <0.001 | 11.39±0.34 | <0.001 | 12.17±0.24 | <0.001 |
| Oxidative stress index (OSI=TOS/TAS) | 5.93±0.09 | <0.001 | 10.86±0.48 | – | 8.25±0.47 | <0.001 | 7.36±0.31 | <0.001 | 7.05±0.12 | <0.001 |
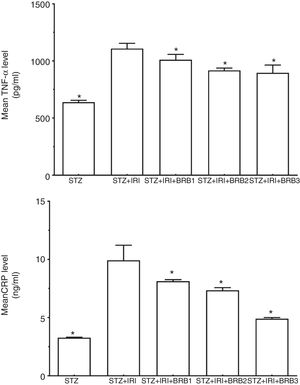
| Tumor necrosis factor alpha (TNF-α) (pg/ml) | 638.98±16.82 | <0.001 | 1108.38±46.02 | – | 1011.43±46.28 | 0.008 | 918.44±19.62 | <0.001 | 895.96±68.23 | <0.001 |
| C-reactive protein (CRP) (ng/ml) | 3.28±0.04 | <0.001 | 9.93±1.29 | – | 8.14±0.13 | <0.001 | 7.34±0.23 | <0.001 | 4.91±0.10 | <0.001 |
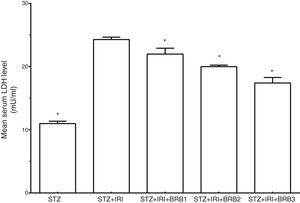
| Lactate dehydrogenase (LDH) (mU/ml) | 10.99±0.36 | <0.001 | 24.28±0.38 | – | 22.14±0.97 | <0.001 | 19.98±0.24 | <0.001 | 17.40±0.87 | <0.001 |
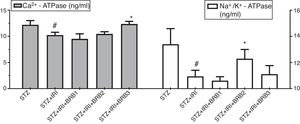
| Calcium triphosphatase (Ca2+-ATPase) (ng/ml) | 12.22±0.79 | 0.001 | 10.22±0.56 | – | 9.49±0.98 | >0.05 | 10.46±0.42 | >0.05 | 12.35±0.52 | 0.001 |
| Sodium potassium adenosine triphosphatase (Na+/K+-ATPase) (ng/ml) | 13.38±1.21 | <0.001 | 10.94±0.47 | – | 10.60±0.31 | >0.05 | 12.29±0.71 | 0.039 | 11.10±0.66 | >0.05 |
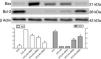
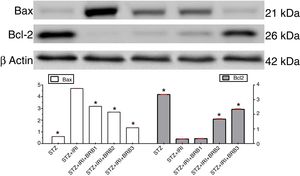
| Bax | 0.48±0.01 | <0.001 | 3.76±0.17 | – | 2.54±0.01 | 0.009 | 2.15±0.01 | <0.001 | 1.08±0.01 | 0.001 |
| Bcl-2 | 4.18±0.01 | <0.001 | 0.39±0.01 | – | 0.42±0.01 | >0.05 | 2.10±0.02 | <0.001 | 2.92±0.03 | 0.001 |
The data are expressed as mean±standard deviation. p value<0.05 was considered significant. All results belong to STZ+IRI compared to the other groups, and p values were represented in the columns.
Serum BUN and creatinine levels which are the markers for kidney functions were evaluated (Fig. 3). The mean BUN and creatinine levels were markedly higher in STZ+IRI group than that of STZ group. Those levels significantly decreased after berberine administration in all berberine administrated groups (p<0.001), except mean serum creatinine level which was not significantly changed between STZ+IRI+BRB1 and STZ+IRI group (p>0.05).
Assessment of oxidative stressThe highest mean TOS was detected in STZ+RIR group (Fig. 4). The levels in berberine administrated groups were statistically lower than that of STZ+IRI group (p<0.001, for all comparisons). The lowest mean total antioxidant status (TAS) was detected in STZ+IRI group (Fig. 4). Berberine administration gradually increased antioxidant status in a dose dependent manner when compared to STZ+RIR group (p<0.001, for all groups).
Oxidative stress index was calculated as ratio of TOS and TAS. OSI in STZ+IRI group was considerably higher than the other groups (Fig. 4). OSI decreased in berberine administrated groups (p<0.001, for all groups). According to these results, berberine markedly suppressed oxidative stress via stimulating antioxidant system.
Assessment of lactate dehydrogenaseThe mean serum LDH level which is a marker for tubular necrosis was higher in STZ+IRI group than that of STZ group (p<0.001, Fig. 5). The levels in STZ+IRI+BRB1, STZ+IRI+BRB2, and STZ+IRI+BRB3 groups were significantly lower than STZ+IRI group (p<0.001, for all of comparisons).
Assessment of inflammation markersThe highest mean TNF-α and CRP levels were detected in STZ+IRI group compared to STZ (p<0.001) and the other groups. The levels gradually decreased in berberine administrated groups including STZ+IRI+BRB1, STZ+IRI+BRB2, and STZ+IRI+BRB3 groups (Fig. 6).
Berberine significantly showed an anti-inflammatory effect against renal ischemia injury via reducing tissue levels of TNF-α and CRP (p≤0.05).
Assessment of apoptosisExpression of pro-apoptotic Bax protein significantly increased but expression of anti-apoptotic Bcl-2 protein decreased in STZ+IRI group compared to the other groups (p<0.001, Fig. 7). IRI markedly caused apoptosis via inducing pro-apoptotic protein, and suppressing anti-apoptotic protein (p<0.001). Berberine administrations at the doses of 100mg/kg and 150mg/kg increased expression of pro-apoptotic Bcl-2 (p<0.001), but berberine at a dose of 50mg/kg in STZ+IRI+BRB1 did not perform any anti-apoptotic effect compared to STZ+IRI group.
Assessment of Ca2+ ATPase and Na+/K+-ATPase enzyme activitiesThe mean tissue Ca2+ ATPase enzyme activity was markedly lower in STZ+IRI group than that of STZ group. The highest mean Ca2+ ATPase enzyme activity was detected in STZ+IRI+BRB3. Berberine administration at a dose of 150mg/kg significantly increased that enzyme activity (p<0.01, Fig. 8). The mean tissue Na+/K+-ATPase enzyme activity was detected as 13.38± 1.21 in STZ group while 10.94±0.47 in STZ+IRI group (p<0.001). The activity of this enzyme in STZ+IRI+BRB2 group was significantly higher than that of STZ+IRI group (p=0.039, Fig. 8).
DiscussionAcute kidney injury (AKI) or renal failure has been described as a rapid dysfunction of the kidney. Ischemia/reperfusion injury is the most important reason of acute renal failure.21,22 Diabetes mellitus is also an important risk factor for the renal failure.4 Animal models have been widely for understanding mechanisms of IRI. It was recorded that IRI has resulted in serious histopathological and biochemical damages in both patients and experimental animals.23–25
BUN and serum creatinine levels are the most commonly used markers evaluating renal function. Significantly increased BUN and creatinine levels are typical indicators of AKI.26–29 We detected significantly increased BUN and creatinine levels in ischemic diabetic group. These levels were decreased in BRB administrated groups. Those decreases might show the beneficial effect of berberine on kidney functions.
Especially re-oxygenation of tissue after hypoxia seriously causes accumulation of free radicals, known as oxidative stress. Both ischemia and hyperglycemia aggravate production of reactive oxygen species (ROS), and it is possible that the two factors precipitate each other.30,31 Clinical and experimental studies showed that diabetes increased the degree of ischemic injury.16,31,32 Measurement of TOS and TAS is a crucial biomarker to evaluate oxidative damage.31–33 Since tissue TOS level was markedly increased in STZ+IRI group we suggest that IRI induces oxidative stress. Conversely since all doses of BRB decreased TOS level but increased TAS level, we suggest that BRB is effective in reducing oxidative stress and damage induced by IRI in diabetic rats.
In the current study, we detected serious histopathological damages including tubular, glomerular, interstitial, and vascular damage in diabetic rats after IRI in accordance with previous studies.4,5,10,28 Proximal and distal tubular damage was more obvious than glomerular damage as previously reported.34–36 Endothelial damage in peritubular capillaries caused by vascular congestion, and leukocyte infiltration was reported after IRI.10,22,24,25 We observed that dilatation and congestion of the capillaries were even for per sample. Mononuclear cell infiltration accompanied by necrosis occupied large area in ischemic group. The glomerular basement membrane in STZ+IRI group was thicker than those of the other groups. BRB, in a dose-dependent manner, improved histopathological findings; the most effective dose was 150mg/kg for tissue healing after IRI. Even the decrease in total pathological score at a dose of 50mg/kg BRB administrated group was evident, it was not statistically significant.
Tubular necrosis was obvious in diabetic ischemic group. LDH is an important indicator for necrosis thus increased levels of LDH in serum indicates plasma membrane damage.36,37 Following IRI, we detected significantly increased LDH levels concomitant with severe tubular damage. Necrotic cell death stimulates inflammatory responses.6,22 Hence inflammation has been observed generally together with necrosis induced by IRI. TNF-α, TGF-β and CRP have been widely used as inflammation markers. The serum concentrations of those markers increase after acute or chronic renal injury associated with diabetes.28,38,39 In the present study TNF-α and CRP level in IRI group were markedly increased in diabetic rats. It was mentioned in previous studies that BRB has anti-inflammatory effect via inhibiting inflammatory markers.40,41 In current study, BRB showed similar anti-inflammatory effect against IRI in all berberine administrated groups via decreasing levels of TNF-α and CRP.
Diabetes and ischemia both, significantly induces apoptotic cell death.25,42 In ischemic tissue, pro-apoptotic Bax protein level significantly increased while anti-apoptotic Bcl2 protein level decreased in diabetic rats. BRB administration at the doses of 100mg/kg and 150mg/kg showed significant anti-apoptotic effect via suppressing anti-apoptotic protein, while BRB administration at a dose of 50mg/kg was not effective as well as other doses. Visnagri et al.28 demonstrated that BRB at the doses of 20mg/kg and 40mg/kg has similar anti-apoptotic effect in non-diabetic rats. During hypoxia, as a result of cellular damage, intracellular calcium level dramatically increases.43 The Ca2+-ATPase(s) enzyme found in the plasma membrane and endoplasmic reticulum has an important role in maintaining the intracellular Ca2+ level.44 In our study Ca2+-ATPase enzyme level significantly decreased after IRI in diabetic rats. We suggest that decrease of Ca2+-ATPase leads to increase of intracellular Ca2+ concentration. Conversely, Visnagri et al.28 have suggested that ischemia induces apoptosis, and Ca2+-ATPase enzyme level increases in ischemic group. We detected decreased levels of Ca2+-ATPase in STZ+IRI group. Increase in intracellular Ca2+ is an indicator of apoptotic cell death.45 Anti-apoptotic Bcl 2 has a crucial function on Ca2+ homeostasis. Bcl-2 overexpression causes decreased Ca2+ releasing from endoplasmic reticulum.45 As we mentioned before, expression of Bcl2 level decreased in ischemic group. Both decreasing of Ca2+-ATPse enzyme and Bcl2 levels indicated that renal tissue cell sustained apoptotic cell death. In contrast to STZ+IRI group, BRB showed anti-apoptotic effect via increasing Ca2+-ATPase enzyme and Bcl 2 protein levels.
The Na+/K+-ATPase enzyme, known as energy-dependent sodium pump, transports Na+ into and K+ out of the cell against their concentration gradients.46 This pump is essential for the maintenance of Na+ and K+ concentrations across the membrane. When this pump stops working or destroys, Na+ accumulates in cytoplasm.42 Tubular epithelial cell polarity thus fluid/ion transport are significantly destroyed after IRI. It has been shown in several organs including kidney, myocardium, brain, hippocampus, etc. that ischemia injury resulted in decrease in the levels of Na+/K+-ATPase enzyme.29,47–50 IRI markedly caused in decline of Na+/K+-ATPase level in diabetic rats, and BRB administration at a dose of 100mg/kg significantly increased that of enzyme level.
ConclusionAs a conclusion berberine administration following ischemia/reperfusion markedly improved histopathological findings, prevented necrotic cell death. Berberine normalized kidney function via decreasing BUN and creatinine levels. Berberine showed also anti-oxidant, anti-apoptotic, and anti-inflammatory effect. According to these data we suggest that berberine at the doses of 100 and 150mg may be used as a potential therapeutic agent against renal ischemic injury.
Conflict of interestAll authors declared that they have no conflict of interest.
FundingThis work was supported by the Bezmialem Vakif University Scientific Research Project Foundation [grant number 6.2017/8].