Interleukin-17A (IL-17A) is a proinflammatory cytokine produced by cells of the immune system, predominantly Th17 and γδ lymphocytes. In this paper, we review the role of IL-17A in the pathogenesis of hypertension and in target organ damage. Preclinical studies in mice have shown that systemic adminstration of IL-17A increases blood pressure, probably by acting on multiple levels. Furthermore, IL-17A plasma concentrations are already elevated in patients with mild or moderate hypertension. Many studies in hypertensive mice models have detected IL-17A-producing cells in target organs such as the heart, vessels and kidneys. Patients with hypertensive nephrosclerosis show kidney infiltration by Th17 lymphocytes and γδ lymphocytes that express IL-17A. In addition, in experimental models of hypertension, the blockade of IL-17A by genetic strategies or using neutralizing antibodies, disminished blood pressure, probablyby acting on the small mesenteric arteries as well as in the regulation of tubule sodium transport. Moreover, IL-17A inhibition reduces end-organs damage. As a whole, the data presented in this review suggest that IL-17A participates in the regulation of blood pressure and in the genesis and maintenance of arterial hypertension, and may constitute a therapeutic target of hypertension-related pathologies in the future.
La interleuquina 17A (IL-17A) es una citoquina proinflamatoria producida por células del sistema inmune, sobre todo por los linfocitos Th17 y los linfocitos γδ. En este trabajo, revisamos el papel de IL-17A en la patogenia de la hipertensión y de la lesión en órganos diana. Estudios en ratones han demostrado que la IL-17A aumenta la presión arterial, probablemente por acciones a varios niveles. Además, las concentraciones plasmáticas de IL-17A están ya aumentadas en pacientes con hipertensión arterial ligera o moderada. Estudios preclínicos sobre hipertensión arterial han detectado células productoras de IL-17A en órganos diana, como corazón, vasos y riñón. En pacientes con nefroesclerosis hipertensiva existe infiltración del riñón por linfocitos Th17 y linfocitos γδ que expresan IL-17A. Además, en modelos experimentales de hipertensión el bloqueo de IL-17A, mediante estrategias génicas, o utilizando anticuerpos neutralizantes, disminuye la presión arterial por acciones sobre la pared vascular y el transporte tubular de sodio y disminuye la lesión en órganos diana. En conjunto, los datos presentados en esta revisión sugieren que la IL-17A participa en la regulación de la presión arterial y en la génesis y mantenimiento de la hipertensión arterial, pudiendo constituir una diana terapéutica en el futuro.
Hypertension is defined as a sustained elevation of blood pressure above the normal range, being a disease prevalent in our society, associated with increased cardiovascular morbidity and mortality.1 Hypertension is a silent disease that can occur without apparent symptoms, but it can cause damage to target organs, such as the cardiovascular system and the kidneys, among others. In addition, these organs are involved in the control of blood pressure, which contributes the research in this field even more difficult. The etiology of essential hypertension continues to not be fully established and is a subject of intense debate among the scientific community. Recently, attention has been drawn to the role of inflammation, autoimmune disorders, and oxidative stress in the development and progression of hypertension, as well as the effect of chronic blood pressure elevation in end-organ damage.2–7
In the 1960s, the first evidences of the participation of the inflammatory response in hypertension were presented.7,8 Supporting this hypothesis, hypertensive patients present elevated serum levels of various proinflammatory cytokines, suggesting that innate immunity, both cellular and humoral, participates in the pathogenesis of hypertension.9–13 Currently, and despite the wide variety of drugs available for its treatment, blood pressure control is not usually optimal in a relevant number of patients, which leads to the existence of important damage in the target organs. For this reason, new therapeutic strategies are necessary to improve the blood pressure control and to protect target organs.
Among the cytokines potentially involved in the genesis and progression of organic damage in hypertension, interleukin IL-17A has acquired an especially relevant role and is one of the most promising therapeutic targets, widely studied in chronic autoimmune and inflammatory diseases, including chronic kidney disease (CKD).3,6,14
This review describes the main general characteristics of the cytokine IL-17A and updates the information on its role in the pathogenesis of hypertension and in the damage of end-organ target.
General characteristics of the IL-17A cytokineIn this section we review the IL-17 cytokines, their receptors, IL-17A-producing cells, and the functions of this cytokine.
IL-17 cytokines and their receptorsThe IL-17 family of cytokines has 6 members, from IL-17A to IL-17F, all of them being involved in the response against infections by pathogens and in chronic autoimmune and inflammatory diseases,15 being the cytokine IL-17A the most studied within the group. This family of cytokines has highly conserved sequences in mammals, such that between the human and murine isoforms of IL-17 there is a sequence homology between 62% in IL-17A and 88% in IL-17B.16 The two members with the highest homology to each other are IL-17A and IL-17F, which can form heterodimers.17 IL-17A, initially called human cytotoxic antigen associated with T-8 lymphocytes, was isolated for the first time in 1993 from a T-cell hybridoma. Although analysis of its cDNA showed that it had some homology with a gene for Herpesvirus samiri, was classified within the group of cytokines because it has an unstable sequence rich in adenylate-uridylate in the 3′UTR region since it is capable of inducing the synthesis of cytokines.18 At the molecular level, IL-17A is a glycoprotein of 155 amino acids and with a molecular weight of 35 kDa, although in general it gives rise to the formation of homodimers linked together by a disulfide bridge.16,19 IL-17 cytokines activate receptors of the same family, the IL-17Rs that consist of 5 members, named IL-17RA through IL-17RE, and that can also form homo- and heterodimers with each other. Thus, IL-17RA is present in most of the dimers that are formed, and in a great diversity of cell types, mostly in immune cells, but the rest of the receptors are specific to specific cell types.20 Likewise, there are also differences in terms of ligand-receptor affinity between the different members, with IL-17A binding with greater affinity to IL-17RA, while IL-17F preferentially binds to IL-17RC.19,21 These affinity differences could help explain the great variability in responses elicited by this versatile family of cytokines.
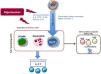
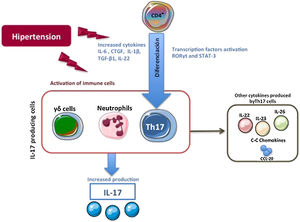
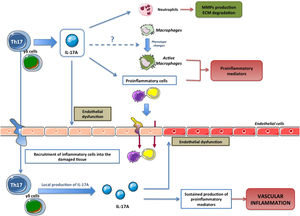
IL-17A producing cellsThe first cells described capable of producing IL-17A were Th17 lymphocytes (CD4+/IL-17A + cells). In general, CD4 + T lymphocytes actively participate in the immune response, in such a way that after antigenic stimulation, naive CD4 + lymphocytes are activated, and they expand and differentiate into the different subpopulations known as T helper (Th).22 Within these Th subpopulations, the effector subtypes Th1, Th2, Th9, Th17, Th22 and follicular Th (Thf) are included, as well as the regulatory T lymphocyte subtype, called Treg, thus broadening the classic view of only two subtypes. Th (Th1 and Th2).23,24 Each Th subtype is classified based on the specific pattern of cytokines that they produce and the different markers that they express.23,24 However, there is great plasticity between the different subtypes, with intermediate cellular phenotypes that are the subject of research, but also controversy.25–28 Differentiation to each Th subtype is driven by a specific combination of cytokines that activate specific transcription factors.29–32 Here, the specific combination of the proinflammatory cytokines IL-1 β, IL-6 IL-21 and or TGF-β1 regulates the differentiation of human naive T lymphocytes to Th17 phenotype. This differentiation is regulated by the activation of both the ROR factor γt (Retinoid related Orphan Receptor γt) and the STAT3 protein (Signal Transducer and Activator of Transcription 3) leading to gene transcription controlling the phenotypic transition to Th17, such as the IL-23R33–37 gene. Once differentiated, Th17 cells mostly secrete IL-17A, but they can also produce IL-22, IL-26 and IL-23, which help stabilize the lineage, or release chemokines that contain the CC motif, such as the chemokine ligand-20 (CCL-20)29,30,33,38,39 (Fig. 1). With this secretion pattern, Th17 cells have as their main function the defense against pathogens in infectious diseases, but they also participate in the pathogenesis of various inflammatory diseases and autoimmune diseases, such as rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis, or chronic inflammatory diseases, including atherosclerosis and hypertension.40–43 In this sense, several factors related to cardiovascular and renal damage, such as the cytokine IL-6, Angiotensin II (Ang II), TGF-β1, or CTGF/CCN2 (connective tissue growth factor/cellular communication network2) participate in the generation of Th17 cells.44–46 IL-17A is produced by cells other than Th17, including γδ lymphocytes (Fig. 1), and probably also by neutrophils, invariant natural killer T cells, CD8 + cells, innate lymphoid cells, and mast cells, although in some Of these cells, there is controversy as to whether they are only capable of storing IL-17A but not producing it, as could be the case with mast cells.30,37,47,48
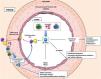
Cellular effects of IL-17AIL-17A generates pro-inflammatory responses that can vary substantially depending on the cell type and pathological conditions.9,10,11,13,49–54 One of the first evidences of the involvement of IL-17A in the inflammatory response showed that in cultured synoviocytes from patients with rheumatoid arthritis, IL-17A increased the levels of IL-6 and IL-8.54 Subsequently, it was observed that IL-17A induced responses in various immune cells, regulating various functions, such as monocyte chemotaxis, and increased production of pro-inflammatory mediators, thus helping to amplify the inflammatory response in damaged tissues11,52,53,55,56 (Fig. 2). The actions of IL-17A in the cells of the immune system are not completely clarified, and it may also participate in the change of macrophage phenotypes,57 and induce the degradation of extracellular matrix by neutrophils, through the regulation of metalloproteinases (MMPs).58
IL-17A in hypertensionHypertensive patients have elevated plasma levels of proinflammatory cytokines, including IL-17A.59–61 A clear association has also been observed between elevated serum levels of IL-17A and a state of pre-hypertension.41,61,62 Along the same lines, circulating levels of IL-17A are also increased in various pathologies associated with hypertension associated with autoimmune diseases, including systemic lupus erythematosus, as well as in pre-eclampsia and chronic transplant rejection.2,10,62,63,64 Furthermore, the high number of circulating IL-17A-producing CD4 + T cells is also increased in hypertensive patients,5 further supporting the hypothesis of the involvement of IL-17A in the development and progression of this disease. Although hypertension does not cause hypernatremia, slight increases in sodium concentration in hypertensive patients can lead to polarization of undifferentiated T cells to Th17 cells, favoring autoimmunity, inflammation and upregulation of the Na-K-2CL cotransporter (NaKCCl).7 In patients with hypertensive nephrosclerosis, we first located IL-17A-producing cells in the kidney, which were identified as Th17 cells (CD4 +/IL-17A +) and γδ65 lymphocytes.
Preclinical studies of IL-17A in hypertension and target organ injuryPreclinical studies revealed ample evidence to support the role of T cells, mainly Th17 cells and its effector cytokine IL-17A, in the pathogenenesis of hypertension.2 The first evidence arose in mice deficient in T and B lymphocytes (RAG1 -/- mice), which were protected from HTN and vascular lesions induced by systemic administration of Ang II. T-cell and B-cell transfer experiments demonstrated that only T cells restore the effects of Ang II in RAG1 −/−66 mice. Other pathological processes in which the participation of Th17 cells has been suggested in pulmonary hypertension associated with hypoxia67 and hypertension caused by calcineurin inhibitor immunosuppressants, such as cyclosporin A and tacrolimus.68
Other preclinical studies have shown the presence of cells that express IL-17A in tissues damaged by hypertension, including the cardiovascular system and the kidneys.2,41,69 Thus, the administration of Ang II to mice increases the infiltrating T lymphocytes that express IL-17A both in the adventitia and in the periadventitial fat of the aorta.41 Subsequently, other studies identified Th17 lymphocytes and γδ lymphocytes, as IL-17A-producing cells in the kidneys and aortas of mice infused with Ang II.69
γδ lymphocytes are involved in the immune response against fungi and pathogens, as well as in autoimmune diseases.70,71 These are unconventional T cells, which recognize many microorganisms and transform host cells, acting as the first line of defense in peripheral tissues.72,73 The IL-17A produced by these cells is mainly involved in antifungal immunity, such as the response to infection by Candida albicans70; and in the initial stages of autoimmune pathologies.74 Lymphocytes γδ producing IL-17A are generated at the periphery, they can be recruited and accumulated in damaged tissues, such as skin, contributing to sustained inflammation, as observed in psoriasis.72 γδ lymphocytes are also the main source of IL-17A in the hypertrophic hearts of mice infused with Ang II.75 In this sense, γδ lymphocytes expressing IL-17A have been observed in the kidney of hypertensive patients.65
These data suggest the possibility of a new therapeutic approach based on the inhibition of these cells to deal with tissue damage associated with hypertension.
IL-17A modulation in experimental hypertensionSeveral experimental studies support the hypothesis of the participation of IL-17A in the pathogenesis of hypertension.4,41,69,76 In this sense, blocking IL-17A by gene deletion of the cytokine or its receptors, or by neutralizing antibodies against IL-17A, lowers blood pressure in experimental models of HT.41,69 However, the deficiency of the IL-17/IL-23 axis did not modify the HTN induced by the combination of DOCA (deoxycorticosterone acetate) and Ang II.77 Preclinical studies demonstrate that IL-17A may directly increase blood pressure, as observed in transgenic mice overexpressing IL-17A specifically in keratinocytes,78 or by administration of IL-17A intraperitoneal (1 µg/day)76 or systemically.65,79 In these last two studies, the dose of IL-17A administered induced serum levels similar to those detected in subjects with blood pressure levels in the range of 120–130/80−89 mmHg,62 values that are currently considered to exceed the optimal levels.80 Other preclinical studies observed an association between the increase in blood pressure induced by IL-17A and the appearance of lesions in target organs such as endothelial dysfunction, structural and functional changes at the vascular or an inflammatory response at the cardiac, vascular and renal levels.67,76,79 Taken together, these data suggest that high circulating levels of IL-17A could contribute both to the development of hypertension and to the induction of target organ damage and, therefore, postulate this cytokine as a potential biomarker and/or therapeutic target.
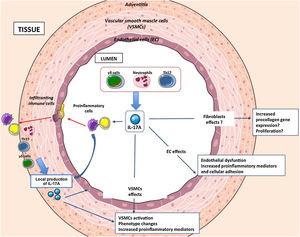
Molecular mechanisms regulated by IL-17A at the vascular levelAmong the mechanisms that could contribute to arterial remodeling associated with hypertension are inflammation, cell number and size (proliferation, apoptosis and/or hypertrophy), changes in the cell phenotype and modifications in the composition of the extracellular matrix that can lead to a vascular fibrosis81 (Fig. 3). IL-17A regulates some of these mechanisms at the vascular level, through which it could regulate hypertension and associated vascular damage.
IL-17A and the inflammatory response in vascular damageInflammation is defined as a non-specific response suffered by a tissue to an attack produced by different mechanical, chemical or biological factors, and whose purpose is to suppress the agent causing the damage and repair the damaged tissue.82 Epidemiological studies have shown that there is an important connection between different chronic inflammatory diseases and cardiovascular diseases. In this sense, the incidence of myocardial infarction is increased in patients with rheumatoid arthritis, systemic lupus erythematosus, or psoriasis.83 These data contribute to highlighting the importance of the inflammatory response in the development and progression of various vascular pathologies, such as vasculitis, aneurysms, hypertension or atherosclerosis.83,84
Recent studies have evaluated the role of vascular inflammation and of specific cytokines and chemokines (eg MCP-1, IL-8, IP-10, and IL-17A) in the onset and development of endothelial dysfunction associated with hypertension.85 IL-17A is a key cytokine in the inflammatory response, included in the cardiovascular system (Fig. 2). In fact, the stimulation with IL-17A increases the expression of multiple pro-inflammatory genes, including mcp-1 and il-6, in cultured murine vascular smooth muscle cells (VSMCs).79 Similarly, IL-17A enhances the pro-inflammatory effect of other cytokines on endothelial cells, VSMCs and macrophages.86 Supporting these findings, the genetic deficiency of IL-17A decreased the inflammatory infiltrate in the murine aortic wall to normal levels.41
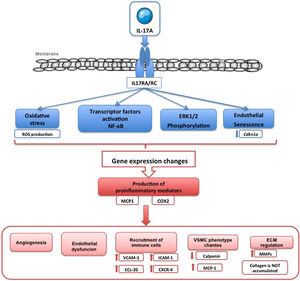
Major proinflammatory mechanisms activated by IL-17A include the production of reactive oxygen species (ROS), of nitric oxide (NO), the activation of the transcription factor factor nuclear kappa B (NF-κB) and regulation of various signaling cascades associated with protein kinases, such as RhoA/Rho-kinase, mitogen-activated protein kinases (MAPKs) or Akt2,14,52,53,55,65,76,78,87 (Fig. 4). NF-κB is a key molecule in the regulation of the inflammatory response, and also in vascular remodeling.88 Activation of this transcription factor has been linked to cardiovascular damage in hypertension, atherosclerosis, cardiac hypertrophy, myocardial infarction or aneurysms formation.88–92 In human umbilical cord endothelial cells (HUVEC) in coculture with T cells (Jurkat cells) IL-17A facilitates endothelial inflammation, by inducing the expression of adhesion molecules ICAM-1 and VCAM-1 and chemokines CCL-20 and CXCR-4 in endothelial cells mediated by ERK1/2 phosphorylation, and therefore promotes the adherence of T cells to HUVEC87 (Fig. 4). A recent study has shown that the overexpression of IL-17A in T cells in mice causes an increase in the production of ROS in circulating cells, vascular dysfunction and perivascular fibrosis compared to control mice.93
Lately it has been shown that the TLR-4 receptor (Toll-like receptor 4) participates in hypertension94 and in the regulation of experimental inflammation in the kidney.95,96 In this sense, mice deficient in TLR-4 are partially protected from AngII-induced blood pressure elevation and the overproduction of ROS, macrophage infiltration and the expression of MCP-1 in the kidney.97 Interestingly, IL-17A blockade considerably decreased renal TLR-4 mRNA overexpression induced by Ang II infusion in mice, suggesting an interconnection between IL-17A and TLR-4 in the regulation of hypertension and kidney injury triggered by Ang II.65
IL-17A and endothelial dysfunctionIL-17A induces endothelial dysfunction and oxidative stress, through the intracellular RhoA/Rho-kinase (ROCK) signaling pathway, by phosphorylating threonine residue 495 of endothelial nitric oxide synthase (eNOS Thr495) in mouse aorta76,78,98,99 (Fig. 4). Furthermore, IL-17A deficient mice are protected of AngII-induced blood pressure elevation, endothelial dysfunction, evaluated by vascular reactivity experiments, and ROS overproduction .41 RhoA pathway/Rho kinase and regulation of NO contribute to IL-17A-mediated hypertension. Some data also support the involvement of IL-17A in preeclampsia and autoimmune diseases associated with hypertension, such as systemic lupus erythematosus and chronic rejection of the allograft.76 Conditional dermal overexpression of IL-17A in keratinocytes is characterized by increased systolic blood pressure associated to systemic endothelial dysfunction, increased ROS formation, elevated circulating inflammatory leukocytes CD11b+ left ventricular hypertrophy, and reduced survival compared to control mice.78 In endothelial cells, IL-17A activates the expression of eNOS and cyclooxygenase-2 (COX-2) and this is associated with angiogenesis, determined as proliferation, apoptosis, migration and tubulogenesis.100
IL-17A and vascular extracellular matrixArterial stiffness and the accumulation of extracellular matrix (ECM) can contribute to vascular remodeling and it can be the cause or the consequence of hypertension, and this issue is still highly controversial.101 Studies on the effect of IL-17A modulation on vascular fibrosis provided variable results. The systemic adminstration of IL-17A in mice induced functional and structural changes in mesenteric resistance arteries (MRAs) in vivo characterized by increased intrinsic vascular stiffness (independent of vessel geometry), which could contribute to the increase in blood pressure, but did not modify the three-dimensional structure of elastin nor the levels of collagens, the main components of ECM in MRAs.79 Along the same lines, and supporting the absence of a profibrotic effect of IL-17A at the vascular level, in experimental stenosis due to partial ligation of the carotid artery characterized by vascular remodeling and increased ECM proteins, the IL-17A gene deletion does not change the percentage of stenosis but it did reduce the exterior remodeling.98 Furthermore, the deletion of IL-17A in experimental atherosclerosis in Apolipoprotein E (ApoE) deficient mice did not modify the area of atherosclerotic plaques or the levels of ECM proteins, such as elastin and collagen.98 Regarding the vascular stiffness induced by the systemic administration of Ang II, IL-17A blockade did not improve the stiffness in the MRAs after 2 weeks of infusion,79 but the gene deletion of IL-17A reversed the changes in stiffness induced by Ang II in the aorta at times longer than 4 weeks.102 At the cellular level, IL-17A did not modify the gene expression of profibrotic factors or ECM components in cultured VSMCs79 (Fig. 4). However, studies in aortic fibroblast cultures showed that IL-17A induces the expression of the collagen gene 3a1.102 Studies in mice deficient in cells γδT or antibodies γδT in the model of AngII-induced hypertension showed lower IL-17A production in the in the heart associated with less cardiac fibrosis .75 In summary, various studies have shown contradictory effects of IL-17A on fibrosis, suggesting that this cytokine has different actions depending on the cell type and the pathology involved.103
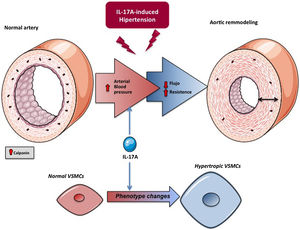
IL-17A, cell growth and phenotype changesIL-17A regulates cell growth in cultured cells, although the effect depends on the cell type. Thus, while IL-17A induces apoptosis in cardiomyocytes,104 it has been postulated as a proliferative and migratory factor in VSMCs,79,105 fibroblasts106 and endothelial cells107. In a context of a tumor microenvironment, IL-17A triggered cell proliferation and epithelial to mesenchymal transition (EMT), evidenced mainly by the inhibition of epithelial markers, such as E-cadherin, and the increase of mesenchymal markers, such as vimentin.108 In this sense, some preclinical studies, in human prostate cancer cell lines109 and in mice treated with a neutralizing anti-IL-17A antibody,110 demonstrated the crucial participation of IL-17A in the development of EMT. Recently, IL-17A produced by neutrophils has been implicated in the progression of gastric cancer by inducing EMT via JAK / STAT3 pathway activation.111 The active participation of IL-17A in cell cycle modulation in a tumor context suggests that it could also trigger effects in a physiological environment. In cultured tubular cells, the role of IL-17A in the regulation of the cellular phenotype, specifically patial EMT has been confirmed, which may contribute to tubulointerstitial fibrosis.109,112 At the vascular level, remodeling involves changes in the VSMCs with a phenotypic transition from the classic contractile cell type to a poorly contractile phenotype, known as the synthetic type. These cellular transformations are associated with changes in the levels of a series of proteins, both cellular and of the secretome, that are characteristic of each phenotype.101,113 In in vivo studies, IL-17A modifies calponin expression in VSMCs of AMRs, which reflects a change in the cellular phenotype79 (Fig. 5). Although it has been classically considered that arterial stiffness is a consequence of increased ECM, mainly due to accumulation of collagen, it has recently been shown that stiffness can also appear in the absence of fibrosis, in this case being regulated by proteins that control contractility of the VSMCs and cell-ECM interactions, including calponin.101 Calponin is an actin-binding protein that regulates contractile functions and stabilization of stress fibers in VSMCs114 and is decreased in various models of hypertrophic vascular remodeling.115,116 The decrease in calponin levels in the vascular cells of the MRAs induced by IL-17A could contribute to increasing vascular stiffness and blood pressure (Fig. 4).
On the other hand, it is important to point out the role that IL-17A plays in promoting the senescence of endothelial cells cultured117 (Fig. 4) and in vivo, according to results obtained in knockout mice for IL-17A or for its receptor.118 Thus, recently, it has been described that a neutralizing antibody to IL-17 reduced the expression of the senescence marker Cdkn1a.119
Potential mechanisms of IL-17A in the control of blood pressure and in target organ injury: actions at the vascular levelThe mechanisms that regulate blood pressure are complex, and involve the participation of different organs and systems. One of the mechanisms that contribute to hypertension is the appearance of structural and functional changes in the arteries (a process known as vascular remodeling), such as a reduction in the diameter of the vessel lumen or an increase in the ratio between the middle layer and the lumen, generating an increase in the pressure exerted by the blood on the wall of the vessels. Vascular remodeling depends on cellular processes such as cell growth, hypertrophy of VSMCs or the overproduction of ECM proteins, such as collagen and fibronectin.120–122 Specifically, remodeling of small-caliber arteries or arterioles participates in the pathogenesis of HT. The vascular remodeling of the MRAs in patients with essential hypertension is characterized by an increase in the thickness of the vascular wall, the mean/lumen ratio and the stiffness of the wall. These changes increase peripheral vascular resistance by increasing blood pressure.123–125 Most of the preclinical studies that have evaluated the role of IL-17A in experimental hypertension have focused on the study of structural and functional changes in the aorta.41,76,78 However, the changes in the aorta would not be enough to explain the increase in blood pressure, suggesting that they could be a consequence of the hypertension itself. In this sense, IL-17A participates in the vascular remodeling ofMRAs, suggesting that this cytokine could be a causative factor of hypertension.79 In mice, the systemic administration of IL-17A to increase circulating levels of IL-17A to values similar to those observed in prehypertensive patients causes an increase in blood pressure associated with structural and functional changes in MRAs, characterized by hypertrophic rinward remodelling and an increase in arterial stiffness.79 In addition, a combination therapy of hydralazine and hydrochlorothiazide, administered when IL-17A had already induced an increase in blood pressure, was able to lower blood pressure, but not to reverse the changes in the mechanical and structural properties of the MRAs induced by IL-17A, suggesting a direct effect of IL-17A on MRAs. Supporting this hypothesis, we demonstrated that a neutralizing antibody to IL-17A normalized vascular remodeling in MRAs induced by systemic administration of Ang II.79 These preclinical studies suggest that the changes induced by IL-17A in the small arteries could be responsible, at least in part, for the increase in blood pressure, demonstrating a new mechanism with which IL-17A could contribute to the development of hypertension (Fig. 5).
Other mechanisms of blood pressure control by IL-17AThe kidney is a key organ in the regulation of blood pressure, and it is also a target for the actions of IL-17.77,126 From the physiological point of view, only the insufficient elimination of salt and water by the kidney can already increase blood pressure in a sustained manner.127 Various cytokines modulate the water and saline balance by altering the sympathetic tone, causing endothelial dysfunction with side effects on renal blood flow or modulating sodium transport along the nephron.128 In this sense, B lymphocytes and dendritic cells can indirectly alter blood pressure by facilitating the activation of T cells. Proinflammatory cytokines of Th1, Th17 cells and macrophages, such as TGF- β1, TNF- α, IFN-γ, IL-1 β and IL-17A increase blood pressure and/or kidney damage.129 At the renal level, IL-17A increases sodium reabsorption through the sodium-proton exchanger type 3 in the proximal tubule and the sodium-chlorine cotransporter in the distal convoluted tubule, contributing to the hypertension.126 Furthermore, deficiency of IL-17A activation suppressed conveyors the distal tubule, in particular of the sodium-potassium cotransporter and the epithelial sodium channel, and decreased renal damage induced by Ang II.126 In mice, the administration of Ang II produced hypertension and reduced the ability to excrete a saline overload, and to activate various sodium transporters in the proximal and distal tubules. However, the hypertensive response to the systemic administration of Ang II was limited in mice deficient in IL-17A, which conserved the renal excretion capacity of sodium overload, mainly due to a lower activity of the sodium transporters of the proximal tubule,130 suggesting that IL-17A interferes with natriuresis induced by increased blood pressure. All these data demonstrate that IL-17A regulates blood pressure by complex mechanisms that act in different tissues and systems.
PerspectivesIn summary, in this manuscript we have reviewed current data implicating IL-17A as a relevant cytokine in the pathogenesis of hypertension and in renal and cardiovascular damage. In preclinical hypertensive models , IL-17A inhibition improved kidney lesions, even when administered therapeutically, as has been observed in a model of diabetic nephropathy.131,132 These data support the hypothesis that IL-17A is an effector cytokine of tissue damage, including in hypertension, and therefore could be considered as a potential therapeutic target in this clinical situation. They are currently clinical trials blocking IL-17A with promising results in hematological proliferative disorders and autoimmune diseases.132 Once their clinical safety has been demonstrated, they could be evaluated as drugs to treat some cases of resistant hypertension and/or prevent damage to the target organ by chonic elevation of blood pressure.
FinancingThis work has been funded by the Spanish Society of Nephrology and by grants from the Carlos III Health Institute (ISCIII) and FEDER European Union Funds (PI17/00119 and PI20/00140 and Renal Research Network (REDINREN): RD16/0009, to MR-O, RS,PI17/01495 to JE),Community of Madrid («NOVELREN»B2017/BMD3751 to MR-O); the José Castillejo grant (CAS19/00133 to RRR-D); «Juan de la Cierva Incorporacion» of the Ministry of Economy, Industry and Competitiveness (MINECO) for SR-M (IJC2018-035187-I); “Call for Dynamization Europe Research 2019” MINECO (EIN2019-1032 94 to MR-O and SR-M);IMPROVE-PD project («Identification and Management of Patients at Risk – Outcome and Vascular Events in Peritoneal Dialysis») of Horizon 2020 Marie Skłodowska-Curie Grant Agreement No. 812699 to MRO, and Fondecyt Chile1160465.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rodrigues-Diez RR, Tejera-Muñoz A, Orejudo M, Marquez-Exposito L, Santos-Sanchez L, Rayego-Mateos S, et al. Interleuquina-17A: potential mediador y diana terapéutica en la hipertensión. Nefrologia. 2021;41:244–257.