Influenza virus or influenza (VI) is an RNA virus belonging to the Orthomyxoviridae family. Vaccination against this virus (VIV) is indicated in groups with a higher probability of VI transmission, developing complications or dying from the disease, such as renal transplant recipients.1 It is considered a safe procedure, however vasculitis after VIV has been described exceptionally.2,3
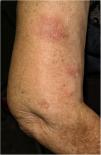
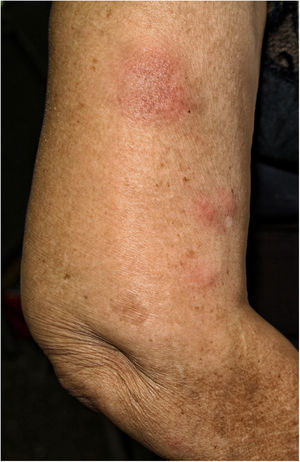
We describe the case of a 77-year-old woman, renal transplant recipient in 1999, due to bilateral urinary reflux and with a functioning graft. Her history included: positivity for total Hb core antibodies and Hbs antibodies and stage II-B diffuse large cell lymphoma treated with chemotherapy in 2006, achieving complete remission until the present. His immunosuppressive treatment for more than five years was rapamycin and mycophenolate mofetil. She was administered VIV, fractionated virus (trivalent: A/Michigan/45/2015 (H1N1)pdm09-like strain; A/Hong Kong/4801/2014 (H3N2)-like strain; B/Brisbane/60/2008-like strain (Victoria lineage)). At 48h after administration she began to feel unwell, complaining of joint pain, diarrhea and fever of 37°C. Four days later, erythematous nodules appeared located on the arms and legs, 1–2cm in diameter, poorly demarcated, painful on pressure, (Fig. 1).
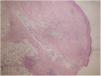
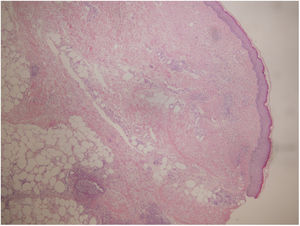
A biopsy was performed showing a moderate perivascular inflammatory infiltrate, with predominance of polymorphonuclear cells, endothelial damage and presence of nuclear dust, located in papillary and reticular dermis with extension to subcutaneous cellular tissue with lobular and septal arrangement (Fig. 2). PAS, Grocott and Giemsa techniques were performed and no microorganisms were identified.
Laboratory tests with CRP, ESR, complement, urinalysis, immunity study (ANCA, cryoglobulins, rheumatoid factor, ANA) and microbiological study (HCV serology) were normal or negative.
With these data, she was diagnosed of leukocytoclastic vasculitis (LV) as a post-VVI reaction.
She was treated with prednisone 30mg/24h in a descending regimen for 20 days with complete resolution of symptoms.
Most vaccines used in European countries with specific vaccination programs, including Spain, are inactivated vaccines: whole, split or subunit vaccines. Around 1–10 % of people receiving VVI have some type of adverse effect, although most of them are mild and self-limited.
Different forms of vaccine-associated vasculitis have been described (Kawasaki disease,4 P-ANCA and C-ANCA positive5), but in the case of VIV the predominant form is cutaneous vasculitis,3 affecting mainly the elderly and women, conditions that were present in the patient described above. However, in most of the cases described, the causal relationship between immunization and VL has not been demonstrated.1,6
Despite what has been published, this pathology is an infrequent adverse reaction, especially in immunosuppressed patients.1 We have found only one case of a renal transplant patient with IgA nephropathy who developed IgA vasculitis after VIV.7 The authors propose the presence of autoimmunity as an etiopathogenic hypothesis. The patient described in this report is a renal transplant recipient due to urinary reflux who suffers a VL with no history of autoimmune diseases. This makes the case even more original since there has not been similar description previously.
The mechanism by which vasculitis secondary to VIV may occur remains unknown. The patient's immunosuppression, rapamycin and mycophenolate mofetil, produce a cytostatic effect on lymphocytes, although the effect it is not absolute. In addition, split virus vaccines (second generation), containing hemagglutinin, neuraminidase and part of the nucleoprotein and M protein, induce humoral immunity and are less reactogenic than whole virus vaccines. Although the inflammatory infiltrate is mainly neutrophilic, there are cases with a predominance of eosinophilic8 or mast cells.9 Therefore, and without ruling out other mechanisms, VIV could provoke the activation of autoreactive B/T cells, despite immunosuppressive treatment, the formation of antibodies and the deposition of immune complexes within the small vessels, which would lead to the activation of the complement system and leukocyte recruitment. However, one could also hypothesize an inflammatory response to some component of the vaccine that targets the endothelium and provokes small vessel vasculitis, causing direct cytopathic damage of the virus to the endothelial cell. In fact, a case of VL after VIV has been described in which an intradermal test was performed, showing a reticulated rash around the inoculation site probably caused by cytotoxic lymphokines.6
In addition, there are some cases of VL in solid organ transplant patients, where sirolimus is considered to be the causative agent. The relationship is established based on the temporality of the symptoms with respect to the introduction of the drug10 in the treatment. Our patient had been on this medication for more than five years, which makes us rule out sirolimus as the etiological agent.
There are no prospective studies on the progression of vasculitis after VIV to a systemic condition with or without renal involvement. For this reason, and taking into account the exceptionality of the case, we believe that this routine should not be restricted in general in renal transplant patients.
In conclusion, to our knowledge we present the first case of VL after VIV in a renal transplant patient.