To the Editor,
There is little information about the actual implementation of Clinical Practice Guidelines on the treatment of anaemia (CPGA) in our country’s peritoneal dialysis (PD) units. Since the first CPGA in 1997,1 scientific societies have published more than 25 guidelines worldwide, although many of them are transpositions of the European2 (EBPG) and American guidelines3,4 (KDOQI).
One of the most controversial aspects has been the target haemoglobin (Hb) levels. Unfortunately, few studies have been published on anaemia in PD. Most major observational studies and all clinical trials on this subject have been conducted in patients on haemodialysis (HD) or patients with chronic kidney disease. As such, the guidelines extrapolate these data to PD without considering the differences between the patients’ profiles and the characteristics of each technique.
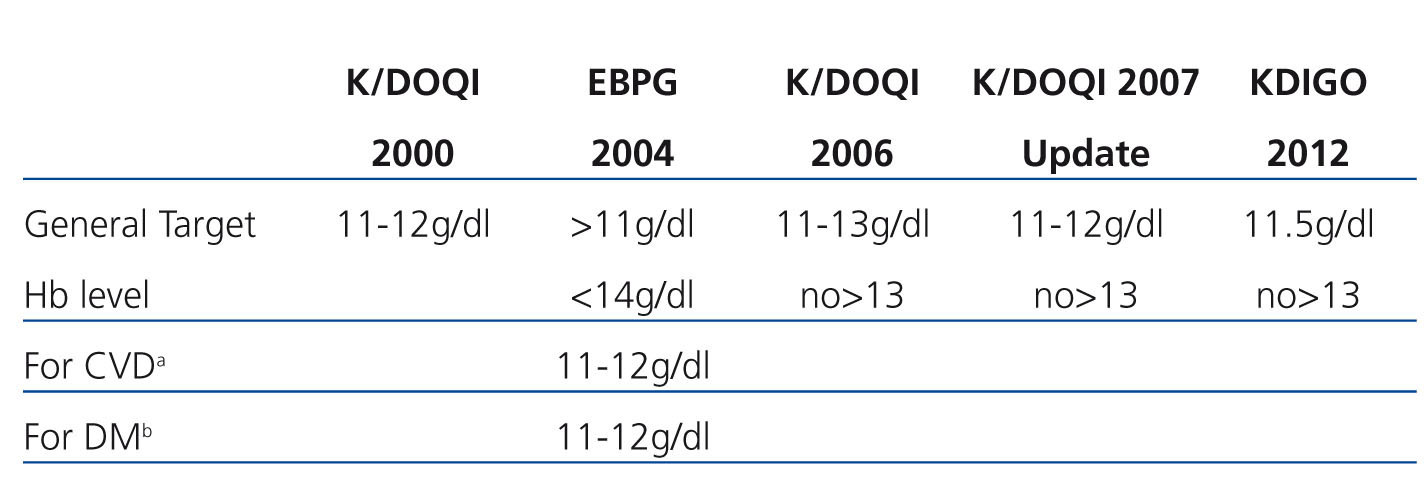
The lower limit target of 11g/dl Hb has actually remained stable from 1997-2009. However, the upper limit has been increasing towards the standardisation of Hb figures, until the EBPG 2004 guidelines, which maintained the absolute maximum limit at 14g/dl, but indicated 12g/dl for patients with previous cardiovascular events. The KDOQI 2006 guidelines recommend not intentionally maintaining Hb>13g/dl and the 2007 update recommends a general range between 11 and 12g/dl. The results of the CREATE, CHOIR and TREAT studies did not show any benefits from standardisation.5
Many of us question the level of knowledge and acceptance of these guidelines, and few data exist regarding their application in the PD of our setting. Most data are taken from observational studies or foreign registries that do not suitably take into account this development over time.
We began our study in September 2011 by surveying the heads of each unit of the Peritoneal Dialysis Centre Group (PDCG). 56% responded with the following relevant facts: only 21.4% of nephrologists say they do not apply any CPGA, 50% apply the most recent (KDOQI or EBPG), although they seem to prefer the European guidelines to the American guidelines (3-1). They also estimate that it takes an average of 3-6 months to apply these CPGA in their regular clinical practice.
The PD Hb control targets depend largely on the management carried out by the patient's nephrologist, since we have effective and safe drugs. Today it is accepted that resistance maintained to treatment with erythropoiesis stimulating agents (ESA) is very rare. In fact, only 1% of HD patients presented a Hb level that is below the target level after 6 months on ESA doses equivalent to 30 000 units erythropoietin/week and this rate may be lower in PD.3 Therefore Hb levels achieved may be a true reflection of the degree of implementation of the current CPGA at any given time.2-4
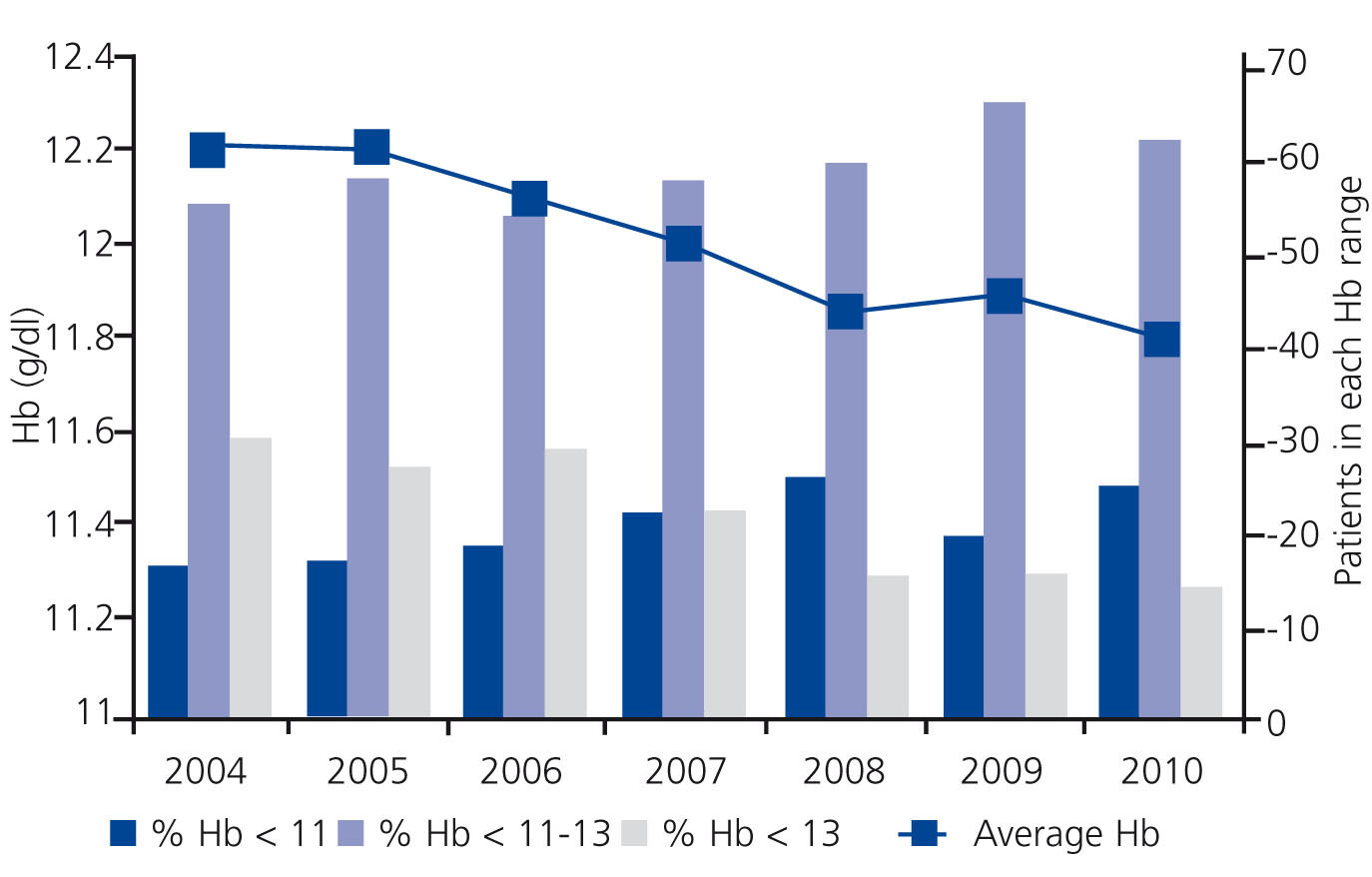
The CPGA has a multipurpose database that collects data prospectively, which has shown to be a representative sample of PD patient management in our setting, the operation of which has previously been described.6 It is an open database which incorporates new incident patients whose follow-up ends with the withdrawal of PD. In our analysis, we included 1166 patients from 2004 to 2010. The characteristics of this group have been described previously, but it is clear that these are younger patients with less comorbidity than their peers on HD. In order to calculate the average Hb level, each patient contributes a single average value from their available Hb figures and all available data for the year is used for stratification by intervals. Figure 1 shows a summary of the change in values. Comorbidy of patients, as measured by the Charlson index, was not significantly different between the different years (3.2 to 3.3) and the age increased from 53.8 in the first year to 56.22 in the last, with a progressive linear trend. There were no significant changes in ESA dose measured by weight.
We have previously published that patients begin PD with better anaemia control than their peers on HD presented by the MAR study.6,7 The failure to achieve targets also seems to be more frequent due to high Hb than to low Hb.6 This may reflect a sense of security in the management of younger and less comorbid patients and patients with less cardiovascular involvement than their counterparts on HD and those who also have a high early withdrawal rate due to transplantation. PD does not subject patients to ultrafiltration, which causes haemoconcentration, and these patients also have a more active and socially integrated life. All this seems to shift the risk/benefit analysis towards higher Hb levels. We did not find data showing that higher levels of Hb are harmful and mortality for this group is below that observed in national registries.6,8
Our data show that the average Hb levels have dropped over the years (11.8g/dl in 2010 vs. 12.2g/dl in 2004, P<.001), and the number of patients with Hb>13g/dl is steadily falling (14.3% vs. 29.6%) at the same time that there is an increase in the percentage of those with Hb<11g/dl (24.9% vs. 16.3%, P<.001) The percentage of patients in the 11-13 range is improving year after year (60.8% vs. 54.1%).
This analysis is significantly limited since we cannot analyse elements that influence anaemia management such as supporting treatments, inflammation and nutrition, but we consider that the data confirm what is described in the survey, constituting a near future and current reference of anaemia management in our PD units and the implementation of the GPCA (Table 1).
It is expected that this trend will continue in the coming years following the recent publication of the 2012 KDIGO guidelines.9 We believe that studies about anaemia, which allow specific targets to be identified for these patients in PD, are necessary.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Evolution of target anaemia levels in chronic kidney disease in the clinical guidelines
Figure 1. Distribution of haemoglobin values in prevalent patients on peritoneal dialysis at the end of every year during the period analysed