Patients undergoing hemodialysis (HD) are characterized by a poor physical condition and a substantial sedentary profile. The implementation of physical exercise programs in the hemodialysis units is usually limited by the inherent safeness and the lack of appropriate resources.
ObjectivesWe aimed to evaluate the impact and safety outcomes of the implementation of an intradialytic physical exercise program (IPE) by a multidisciplinary team (physiotherapist and nursing assistant) in the physical condition of the patients.
Material and methodsThis six months single-centre and experimental pre-post prospective study was carried out in 34 patients. A two day-week combined IPE intervention was implemented. The cardiopulmonary capacity (6MWT), muscular strength (HG, leg dynamometry and 10STS), body composition (bioimpedance) and coordination capacity (Timed Up and Go test) was assessed at the beginning and at the end of the study. Safety was evaluated by means of the number of issues regarding the vascular access, the hemodynamic stability as well as the vascular refilling profile (RBV) in each session. The adhesion to the program was also registered. Additionally, analytical parameters were recorded.
ResultsThe adhesion to an IPE program was high (70.8%). A significant improvement of the cardiopulmonary capacity (6MWT average increase 47 m; p < 0.001), superior limbs (HG average increase of 1.6 kg; p = 0.007) as well as the lower extremities (10STS; p = 0.003; dynamometry p < 0.05). Regarding safeness, there were no incidences neither significant difference in the RBV.
ConclusionsA combined IPE may contribute to the improvement of the physical condition of the patients as well as ensures a safe development of the HD treatment. We suggest a multidisciplinary team in order to efficiently establish an IPE program.
Los pacientes en hemodiálisis (HD) suelen tener una condición física mermada y elevado sedentarismo. La consolidación de programas de ejercicio físico en las unidades de diálisis está limitada por barreras como la seguridad inherente a estos programas y la falta de recursos.
ObjetivosEvaluar la eficacia y seguridad en la implantación de un programa de ejercicio físico intradiálisis (EFI) sobre la condición física de los pacientes con un equipo multidisciplinar (fisioterapeuta y auxiliares de enfermería).
Material y métodosEstudio cuasiexperimental pre-post unicéntrico prospectivo de 6 meses en 34 pacientes. Intervención con EFI combinado 2 días a la semana, evaluándose de forma basal y final la capacidad cardiorrespiratoria (6MWT), fuerza muscular (HG, dinamometría de cuádriceps y 10STS), la composición corporal (bioimpedanciometría) y la capacidad coordinativa (Timed Up and Go test). La seguridad se valoró registrando las incidencias relacionadas con el acceso vascular, la estabilidad hemodinámica y el perfil de rellenado vascular (RBV) durante las sesiones. También se registró la adherencia al programa así como parámetros analíticos habituales.
ResultadosLa adherencia al programa de EFI fue elevada (70,8%). Se constató una mejora significativa de 47 m (p < 0,001) en el 6MWT; un incremento medio de 1,6 kg (p = 0,007) en el HG para la fuerza de extremidades superiores y en las extremidades inferiores (10STS p = 0,003; dinamometría p < 0,05). Respecto a la seguridad, no se detectaron incidencias ni diferencias significativas en el RBV.
ConclusionesUn programa de EFI combinado favorece la mejora de la condición física de los pacientes en HD sin comprometer la seguridad del tratamiento. Es aconsejable un equipo multidisciplinar para implantar y dar continuidad eficazmente a un programa de EFI.
Currently, chronic kidney disease is affecting one in 7 adults in Spain, of which 40% require renal replacement therapy (RRT) through hemodialysis (HD). In the last decade, kidney disease has increased by 20%, mainly due to the aging of the population and changes in lifestyle.1 This report describes an increasingly aging population that undergoes HD and that in a very high percentage is multi-pathological and with marked physical inactivity and sedentary behavior.
These patients, due to the consequences of their own kidney failure, RRT and their advanced age, show a decrease in their physical condition that is manifested with a reduced cardiorespiratory capacity, which may be around 60–65%2,3 below the healthy peer population, along with decreased muscle mass and strength. They also show greater difficulty in the development of activities of daily living. This situation makes the HD patient to have greater inactivity and increase their sedentary behavior, which is enhanced by the characteristics of the treatment they receive. In addition, the physical condition of patients deteriorates as they are maintained in HD, and at the same time the level of physical activity also tends to decrease, reducing the survival of those with more sedentary habits.3
Different factors such as a reduced physical condition, multiple pathologies, advanced age and an increase in sedentary behavior translate into an increase in cardiovascular risk, all of which contribute to the main cause of death in patients with chronic renal failure.
Since the early 1980s, countries such as the United States began to implement physical exercise programs during the HD session. Despite the scientific evidence regarding the benefits that it entails at a functional, physiological and psychological level, these programs are not being established in a generalized way nor are they combined at the same level with the dietary-nutritional recommendations.
The exercise carried out in studies with HD patients is mainly aerobic or combined (aerobic and muscular strength), although the importance of strength work is currently being verified due to the changes, in structure and function, experienced by the musculature of these patients.4
The interventions with physical exercise in HD patients are usually of two different modalities: intradialysis, executed at the same time as the HD session, and interdialytic or outpatient, carried out outside the HD session, either directed by a professional or by the same patient at home under previous guidelines. Both modalities are associated with an improvement in cardiorespiratory capacity and muscular strength, although different studies show that the intradialysis modality ensures greater adherence and safety of these programs.5,6
The objective of our study is to evaluate the efficacy of the implementation of a combined intradialysis physical exercise program by a multidisciplinary team on physical condition as well as to confirm the safety of this intervention during the HD session.
Material and methodsDesignQuasi-experimental pre-post single-center prospective study of 6 months duration approved by the Ethics Committee of our hospital and carried out in accordance with the standards of the Declaration of Helsinki.
SubjectsPatients were recruited from the Dirac dialysis center (Diàlisi i Recerca Aplicada Clínic), an outpatient HD center at Hospital Clínic de Barcelona.
The selection of patients was made under the following inclusion criteria: being over 18 years of age, on regular HD for a minimum of 3 months, stable vascular access (femoral PTFE discarded) and stable clinical situation. All voluntarily signed the informed consent.
Different demographic variables were recorded such as age, sex, in addition to the Charlson comorbidity index, time in HD treatment, duration of the HD session, vascular access, etiology of kidney failure, and the following medication: antihypertensives, beta-blockers and erythropoietin (EPO).
AssessmentA baseline and final assessment (at 6 months) of 3 of the components of physical condition and coordination capacity was carried out. Likewise, some analytical parameters were determined, the dose of HD using Kt and vital signs were monitored before and after the practice of intradialysis physical exercise (IPE). At the same time, possible incidents during the session were also recorded.
Validated tests were used for the evaluation of the physical condition.
The week before, the patient was informed of the day on which the assessment would be performed prior to the start of the intervention. At that time, he was explained about what consisted the tests that he was going to perform and how he had equipped himself. Any doubts and concerns that the patient may had were solved.
The field tests were always carried out by the physiotherapist in the same HD center, in the gym that it was equipped with, before starting the HD session. The final assessment was performed on the same day of the week as the baseline assessment to avoid the influence of the day of the week on the difference in weight gain between sessions. The different tests were run in the same order.
Physical conditionThe tests chosen for each parameter were the following:
- 1
Cardiorespiratory capacity
6 min Walking Test (6MWT): in this test the patient must walk the maximum distance possible during 6 min without running or jogging. The examiner reports on the time elapsed according to the protocol of test7 and, at the end of 6 min, the person must stop to be able to count the meters achieved. For this study it was used, a 15 m long corridor.
- 2
Muscular strength
Hand grip (HG): it measures the grip strength of the hand. In our study, the patient's position was as follows: sitting with a 90° flexion of elbow and the forearm in a neutral position with the arm close to the body and without resting on the armrest. In the case of patients with AVF, the test was performed in the contralateral limb and those with catheters, in the dominant limb. A prehensile dynamometer (Hydraulic Hand Dynamometer Seahan) was used. The test was performed in triplicate with a one minute break between attempts. The result was obtained from the mean of the 3 attempts.8
Quadriceps Dynamometry – Analytical method to measure isometric strength of the quadriceps. The patient is placed in a sitting position with the arms crossed over his chest and without supporting his back. A tape attaches the distal third of the leg to the dynamometer. Then, the patient extends the knee with maximum force. We used the hydraulic push/pull dynamometer (Baseline®). The test was performed in triplicate on both legs with a one minute rest between attempts. The mean of the 3 results was obtained.
10 Sit-to-Stand test (10STS): allows to measure the functional strength of the lower extremities. The patient must get up and sit down from a chair (42 °C in height in our study) 10 times with the arms crossed on the chest in the shortest possible time. The chair had no armrests and was leaning against the wall. The total time in seconds spent in the exercise9 were recorded.
- 3
Body composition
A spectroscopic bioimpedanciometry device (BCM Body Composition Monitor, Fresenius Medical Care, Germany) was used to measure body composition.
Bioimpedance measurement was performed before starting the middle week session. The patient was stretched out on the HD chair, without metal objects and at rest for a minimum of 5 °C min. The electrodes were placed (2 on the back of the hand and 2 on the back of the foot, creating a closed circuit) on the half of the body contralateral to AVF and, in the case of a catheter, indistinctly in one half body or another.
Taking advantage of the monthly measurement performed on the patients, the following data were extracted: height, weight, body mass index (BMI), cell mass (BCM), lean mass index (LMI), fat mass index (FMI), overhydration (OH), extracellular water (ECW), extra- and intracellular ratio (E/I) and the phase angle 50 Khz (°).
- 4
Coordination ability: ability to react and balance
Timed Up and Go test: the patient starts from a sitting position and when the examiner gives a verbal signal they must get up, walk 3 m as fast as possible, turn around a cone and sit back in the chair. The seconds used to do it are recorded.10
Analytical parametersThe monthly and quarterly analytical controls of parameters that were routinely performed in the patients were recorded: C-reactive protein (CRP), albumin, triglycerides, creatinine, total cholesterol, glucose, sodium, potassium, LDL-cholesterol, HDL-cholesterol, hematocrit, hemoglobin, transferrin, prealbumin, calcium, phosphorus, magnesium, chlorine, protein catabolism rate (nPCR) and urea nitrogen (BUN).
Likewise, the data corresponding to the dose of HD (Kt) corresponding to the date of the initiation of the intervention and after 6 months, once the intervention was completed, were collected.
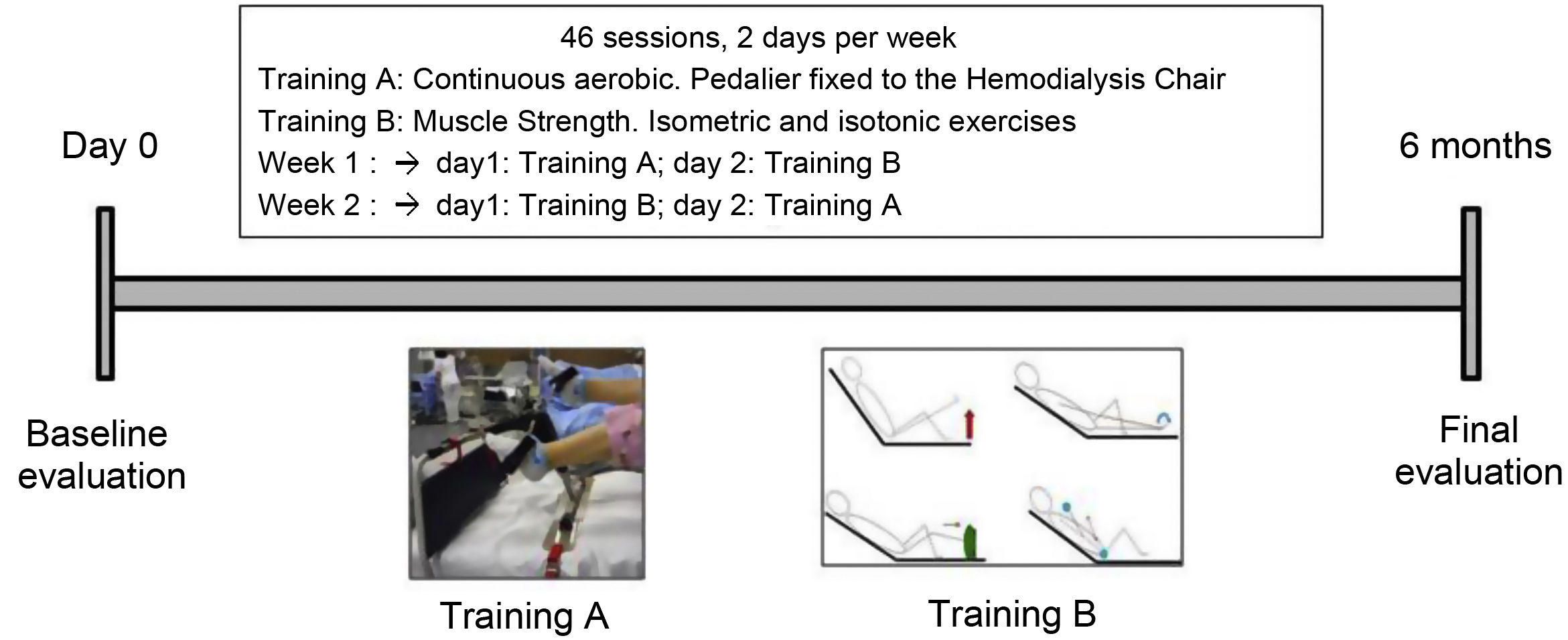
InterventionDuring 6 months, patients performed a combined IPE program where cardiovascular endurance and muscular endurance strength were performed. There were a total of 46 sessions distributed in 2 weekly trainings that were carried out during the second hour of HD. The sessions lasted between 25 and 40 min. The IPE was individualized for each patient based on the results of the baseline assessment and it was supervised one day by the physiotherapist and another day by the auxiliary nursing care technicians (TCAE). The TCAE team was previously instructed in the explanation and supervision of strength training, ensuring good ergonomics for the patient during the execution of both training sessions.
Each week, patients performed one cardio training session and another training session of resistance strength. To ensure the presence of both professionals during the development of both sessions the order of execution of the cardiovascular and strength session was alternated from one week to the next (Fig. 1).
In the cardiovascular endurance session, a continuous aerobic work was done using a bottom bracket (Apex Medical Corp) firmly attached to the dialysis couch. The patients had to pedal at a moderate intensity for 30 min. During the first sessions, depending on the baseline physical condition of the patient, there were one or two rest periods of 1–3 min, until the patient was able to reach the 30 min without stop.
The resistance strength session included isotonic and isometric exercises. The number of repetitions and / or series was increased as the patient tolerated it. The patients began doing one or 2 series of 15 repeats of each exercise until the completion 3 series of 15 repeats. Subsequently, it was introduced material in the exercises such as elastic bands, weights, dumbbells, to the patients who required it.
The intensity of the sessions was adjusted based on the patient's own perception of effort using the classic Borg scale (location of effort between 6 and 20, with 20 being a maximum effort). The patient was asked to feel a perception of 12–15/20 to perform the moderate effort as recommended.
Blood pressure and heart rate (HR) were recorded before starting each IPE session. The patient was also asked about the effects and perceptions of the previous session: excessive fatigue, the manifestation of some discomfort, added pain and/or hypotension, among others. At the end of each session, the constants were taken again as well as the existence any incidents during the course of the training or if there had been any reason to be interrupted: extravasations in the vascular access, discomfort of the patient during the execution of the exercise or presence of any musculoskeletal pain. Each patient had a template with the record of pre-post IPE session constants and the incidents that occurred.
Another parameter recorded during each session with IPE was the monitorization of the percent reduction in the relative plasma volume (% RBV), to evaluate the behavior of the vascular filling using the BVM device (Blood Volume Monitor) arranged in the arterial line of the dialysis monitor.
As far as adherence to the program, those cases that had performed less than 75% of the total sessions were removed since it meant a too long interruption of the program, and the fact of not having continuity with the exercise denoted restarting the assessment by the possible loss of adaptations achieved.
Statistic analysisThe normality of each variable was determined using the Shapiro-Wilk test.
The results are expressed as the arithmetic mean ± standard deviation (SD) for the normal variables and as median and range for the non-normal variables.
For the analysis of statistical significance, the t-Student test was used to compare means of paired data (95% CI) for normal variables, and the Wilcoxon test for non-normal variables. SPSS version 19 software was used.
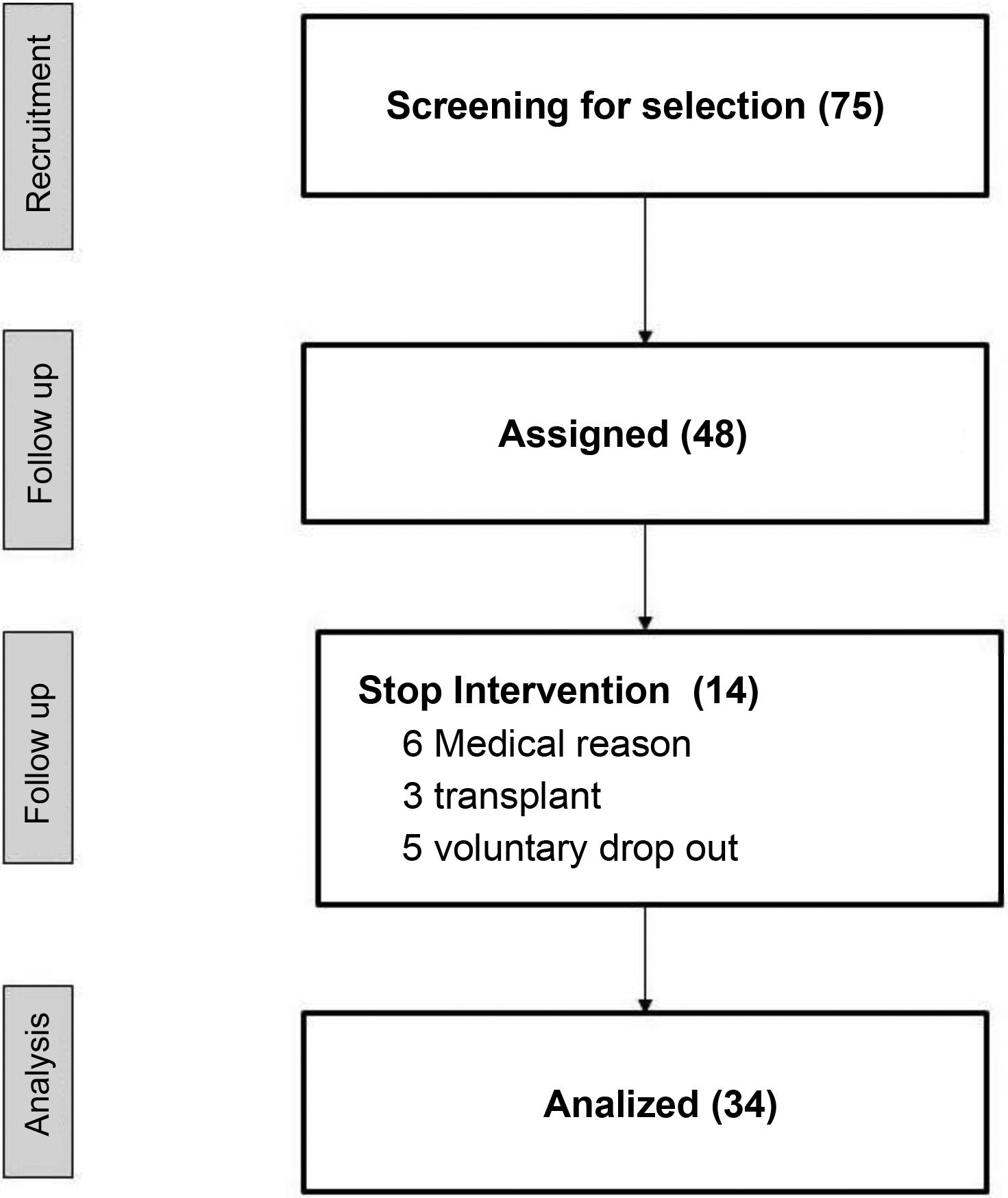
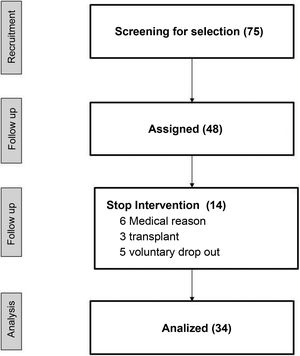
ResultsOf a total of 75 patients of the center, 48 were preselected to perform the study, and 34 finished the study. Table 1 shows the demographic data and the medication. The reasons for not completing 75% of the total number of sessions scheduled were: unstable medical situation, refusal to continue the study and transplantation (Fig. 2).
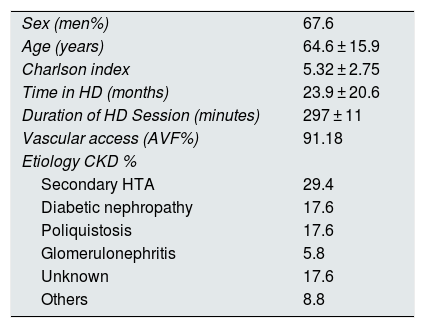
Demographic data.
| Sex (men%) | 67.6 |
| Age (years) | 64.6 ± 15.9 |
| Charlson index | 5.32 ± 2.75 |
| Time in HD (months) | 23.9 ± 20.6 |
| Duration of HD Session (minutes) | 297 ± 11 |
| Vascular access (AVF%) | 91.18 |
| Etiology CKD % | |
| Secondary HTA | 29.4 |
| Diabetic nephropathy | 17.6 |
| Poliquistosis | 17.6 |
| Glomerulonephritis | 5.8 |
| Unknown | 17.6 |
| Others | 8.8 |
| Medication | Basal | 6 months | p |
|---|---|---|---|
| Antihypertensives (%) | 91 | fifty | 0.340 a |
| EPO (%) | 94 | 91 | 0.937 a |
| Beta-blockers (%) | 29 | 29 |
EPO: erythropoietin; AVF: internal arteriovenous fistula; HD: hemodialysis; HTN: hypertension; CKD: chronic kidney disease.
Adherence to the program varied according to the patient. A 17.6% of patients were able to complete the entire program, 73.6% of the participants executed between 85 and 95% of the total sessions, and 8.8% finished a 75% of the interventions. The main reasons for lack of adherence were the following: patient discomfort (respiratory or musculoskeletal problems), not coming to the center (medical visits and admissions) and problems of puncture of vascular access prior to the start of the IPE session.
In relation to potential changes in medication administration over the course of 6 months, there were no significant differences in antihypertensive medication (p = 0.340) or EPO (p = 0.937).
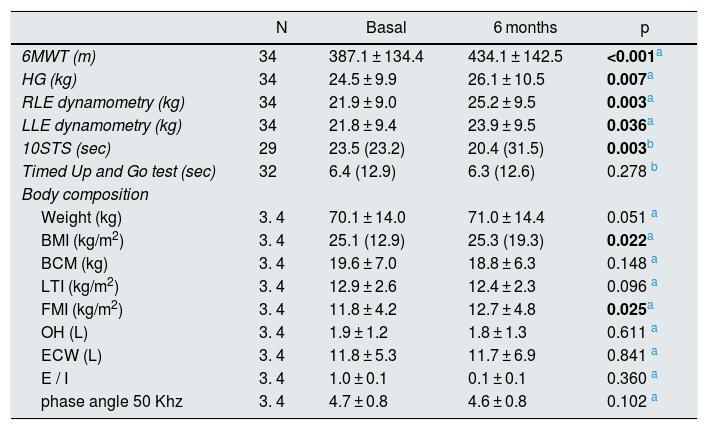
Table 2 shows the results obtained at baseline and final assessment of the physical condition components.
Baseline and final results of the assessment of physical condition.
| N | Basal | 6 months | p | |
|---|---|---|---|---|
| 6MWT (m) | 34 | 387.1 ± 134.4 | 434.1 ± 142.5 | <0.001a |
| HG (kg) | 34 | 24.5 ± 9.9 | 26.1 ± 10.5 | 0.007a |
| RLE dynamometry (kg) | 34 | 21.9 ± 9.0 | 25.2 ± 9.5 | 0.003a |
| LLE dynamometry (kg) | 34 | 21.8 ± 9.4 | 23.9 ± 9.5 | 0.036a |
| 10STS (sec) | 29 | 23.5 (23.2) | 20.4 (31.5) | 0.003b |
| Timed Up and Go test (sec) | 32 | 6.4 (12.9) | 6.3 (12.6) | 0.278 b |
| Body composition | ||||
| Weight (kg) | 3. 4 | 70.1 ± 14.0 | 71.0 ± 14.4 | 0.051 a |
| BMI (kg/m2) | 3. 4 | 25.1 (12.9) | 25.3 (19.3) | 0.022a |
| BCM (kg) | 3. 4 | 19.6 ± 7.0 | 18.8 ± 6.3 | 0.148 a |
| LTI (kg/m2) | 3. 4 | 12.9 ± 2.6 | 12.4 ± 2.3 | 0.096 a |
| FMI (kg/m2) | 3. 4 | 11.8 ± 4.2 | 12.7 ± 4.8 | 0.025a |
| OH (L) | 3. 4 | 1.9 ± 1.2 | 1.8 ± 1.3 | 0.611 a |
| ECW (L) | 3. 4 | 11.8 ± 5.3 | 11.7 ± 6.9 | 0.841 a |
| E / I | 3. 4 | 1.0 ± 0.1 | 0.1 ± 0.1 | 0.360 a |
| phase angle 50 Khz | 3. 4 | 4.7 ± 0.8 | 4.6 ± 0.8 | 0.102 a |
Data expressed as mean ± SD or median y (range).
6MWT: Six Minutes Walking test ; 10STS: 10 Sit-To-Stand tes t; BMI: body mass index; BCM: cell mass; SD: standard deviation; ECW: extracellular water; E / I: extra- and intracellular ratio; RLE: right lower extremity; LLE: left lower extremity FMI: fat mass index; HG: hand grip LTI: lean mass index; OH: overhydration.
Bold: statistically significant values.
Cardiorespiratory capacity significantly improved, with an average increase of 47 m (p < 0.001) in the 6MWT test.
All participants increased of hand grip (HG) strength by a mean of 1.6 kg (p = 0.007).
Regarding the muscular strength of the lower extremities, it was observed a global decrease in the execution time of the 10STS (p = 0.003). The dynamometry of the extension force of the quadriceps, increased by 3.3 kg (p = 0.003) in the right limb and 2.1 kg ( p = 0.036) in the left.
In the Timed Up and Go test, it was observed a decrease in completion time at the end of the study although the difference did not reach statistical significance (p = 0.278).
Regarding body composition, it was detected a significant increase in BMI (p = 0.022) and FMI (p = 0.025). The values of the other registered parameters did not present statistically significant differences.
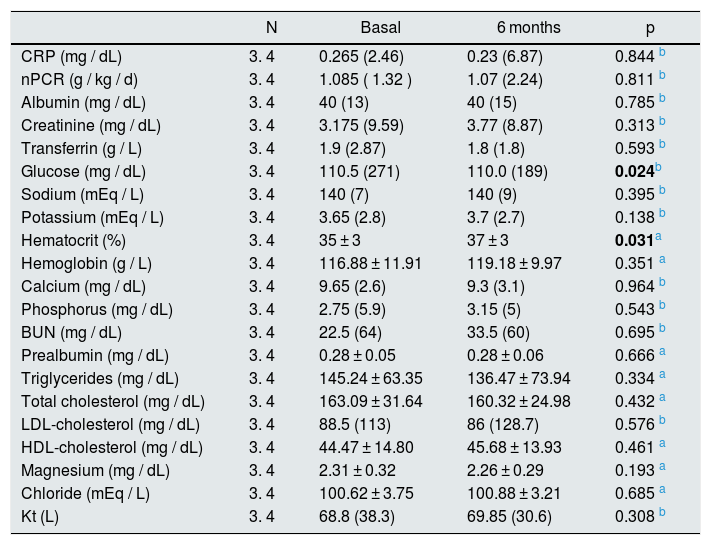
As shown in Table 3, serum glucose decreased (p = 0.024) and hematocrit values, increased by 2% (p = 0.031).
Results of the baseline and final analytical parameters.
| N | Basal | 6 months | p | |
|---|---|---|---|---|
| CRP (mg / dL) | 3. 4 | 0.265 (2.46) | 0.23 (6.87) | 0.844 b |
| nPCR (g / kg / d) | 3. 4 | 1.085 ( 1.32 ) | 1.07 (2.24) | 0.811 b |
| Albumin (mg / dL) | 3. 4 | 40 (13) | 40 (15) | 0.785 b |
| Creatinine (mg / dL) | 3. 4 | 3.175 (9.59) | 3.77 (8.87) | 0.313 b |
| Transferrin (g / L) | 3. 4 | 1.9 (2.87) | 1.8 (1.8) | 0.593 b |
| Glucose (mg / dL) | 3. 4 | 110.5 (271) | 110.0 (189) | 0.024b |
| Sodium (mEq / L) | 3. 4 | 140 (7) | 140 (9) | 0.395 b |
| Potassium (mEq / L) | 3. 4 | 3.65 (2.8) | 3.7 (2.7) | 0.138 b |
| Hematocrit (%) | 3. 4 | 35 ± 3 | 37 ± 3 | 0.031a |
| Hemoglobin (g / L) | 3. 4 | 116.88 ± 11.91 | 119.18 ± 9.97 | 0.351 a |
| Calcium (mg / dL) | 3. 4 | 9.65 (2.6) | 9.3 (3.1) | 0.964 b |
| Phosphorus (mg / dL) | 3. 4 | 2.75 (5.9) | 3.15 (5) | 0.543 b |
| BUN (mg / dL) | 3. 4 | 22.5 (64) | 33.5 (60) | 0.695 b |
| Prealbumin (mg / dL) | 3. 4 | 0.28 ± 0.05 | 0.28 ± 0.06 | 0.666 a |
| Triglycerides (mg / dL) | 3. 4 | 145.24 ± 63.35 | 136.47 ± 73.94 | 0.334 a |
| Total cholesterol (mg / dL) | 3. 4 | 163.09 ± 31.64 | 160.32 ± 24.98 | 0.432 a |
| LDL-cholesterol (mg / dL) | 3. 4 | 88.5 (113) | 86 (128.7) | 0.576 b |
| HDL-cholesterol (mg / dL) | 3. 4 | 44.47 ± 14.80 | 45.68 ± 13.93 | 0.461 a |
| Magnesium (mg / dL) | 3. 4 | 2.31 ± 0.32 | 2.26 ± 0.29 | 0.193 a |
| Chloride (mEq / L) | 3. 4 | 100.62 ± 3.75 | 100.88 ± 3.21 | 0.685 a |
| Kt (L) | 3. 4 | 68.8 (38.3) | 69.85 (30.6) | 0.308 b |
Data expressed as mean ± SD or median y (range).
BUN: urea nitrogen; SD: standard deviation; Kt: dose of HD; nPCR: protein catabolic rate; CRP: C-reactive protein.
Bold: statistically significant values.
The dose of HD pre-post increased 1.2 L, but it was not statistically significant (p = 0.308).
In relation to exercise tolerance and cardiac effort, a 79% of the patients increased their HR (the average difference pre-post-training of each of the session) by 4 beats per minute, while a 21% of patients reduced their HR by an average of less than one beat per minute at the end of the IPE session.
The mean variation in blood pressure pre- and post-IPE in each of sessions was of 3.3 mmHg for systolic and 1.6 mmHg diastolic blood arterial pressure.
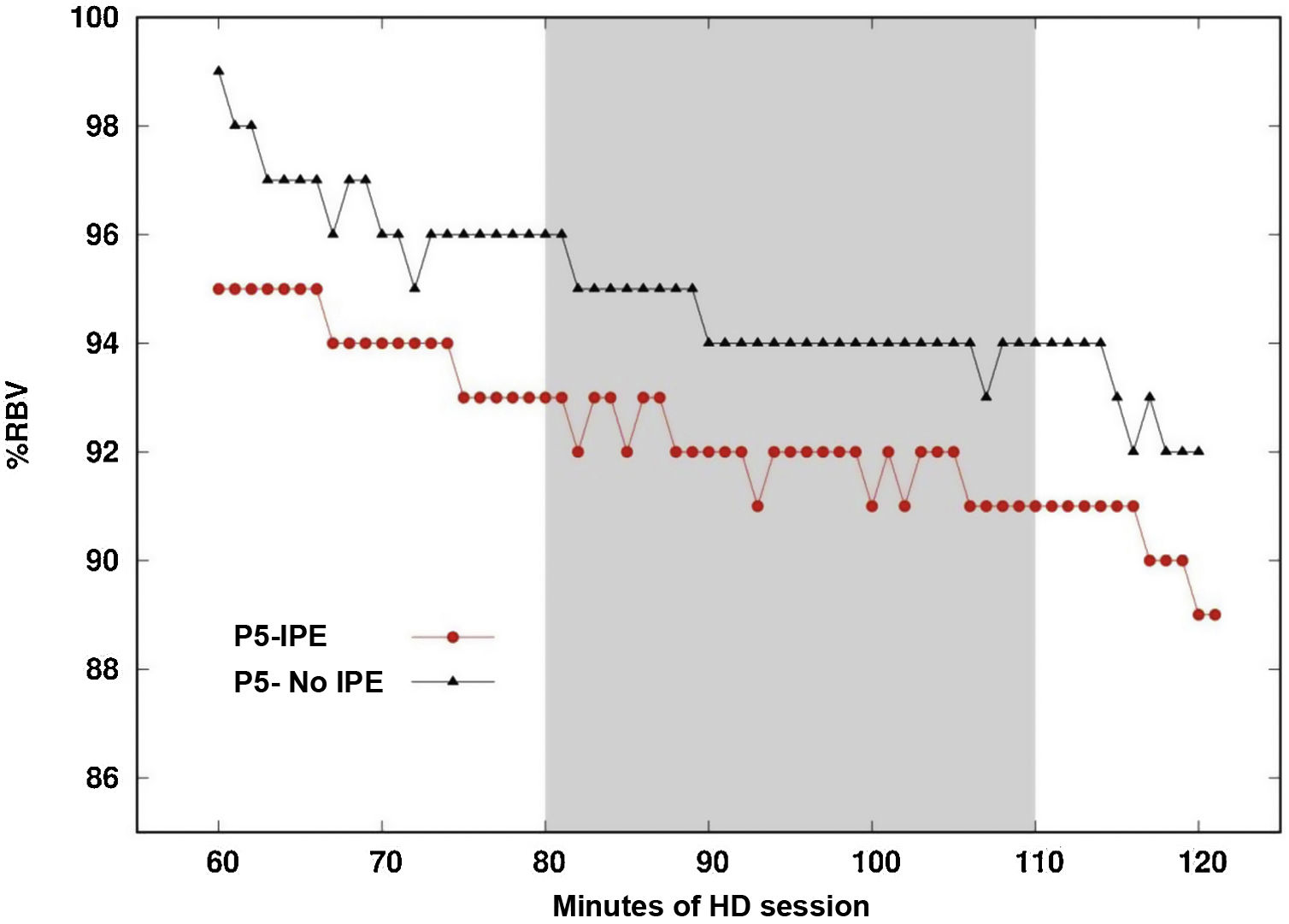
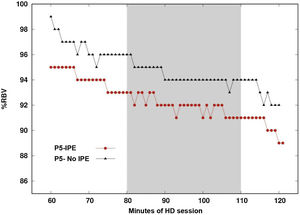
Fig. 3 shows the RBV (Relative blood volume) profile of the same patient during one day with IPE and another day without IPE. The difference between the maximum and minimum value of the RBV percentage averaged −4.38% on the day the patient underwent IPE and −4.45% on the day that they did not perform IPE, this difference being not significant (p = 0.735).
Vascular refilling profile on a day of intradialysis physical exercise and on a day of no exercise.
Vascular filling profile during the second hour of hemodialysis. Comparison on one day of IPE regimen (intervention time marked by the gray band) and one day without IPE regimen for the same patient.
There were no incidents that could have caused the IPE session to be interrupted. During the 6 months of study, there were no vascular access problems (no extravasation or accidental needle removal) caused by exercise in any of the participants. Likewise, the patients did not report musculoskeletal pain or discomfort during the execution of the exercises, neither on the day of cardiovascular training nor on the day of resistance training. Similarly, the patients did not present any symptomatic hypotension caused by the IPE.
DiscussionThe results of this study in HD patients suggest that the practice of combined IPE could contribute to a significant improvement of the cardiorespiratory capacity and the muscular strength of the upper and lower extremities. Likewise, it is clear that the IPE sessions are safe, without altering the hemodynamic status of the patient or affecting the functioning of the vascular access.
Since the early 1980s, countries such as the United States began to implement IPE programs; however, these programs are not being used as widely as is practiced in patients with cardiac and/or respiratory disease.5 Although physical exercise is highly recommended in the HD population to reduce cardiovascular risk, improve physical condition and reduce sedentary behavior, it is not a common practice in HD centers, and have not been widely implemented as part of the comprehensive care of patients in HD, despite being described as a recommendation in the KDIGO guides.11 This is probably due to the fact that there are clear barriers not only among patients, mainly fatigue on the day of HD, fear of injury or lack of time,12,13 but also in health personnel due to the limited knowledge about the type of exercise that can be done, material required and the possible complications that may arise. The end result is that prescription of these activities by nephrologists are infrequent.14
IPE programs are often established as an intervention within pilot studies, but thereafter it is difficult to implement as standard practice within the HD session due to lack of resources. That’s way the importance of putting together a good professional network responsible for carrying out the program15 and ensure its successful continuity. In our study, we had a part-time physiotherapist, assessing the physical condition and prescribing and planning the IPE program as well as supervising it jointly with the TCAE team. Given the distribution of tasks at the beginning of the HD session, it was opportune to involve the TCAE team to support the execution of the program. It would also be valid the participation of volunteers for the execution of this task, as long as they receive the necessary training. The role of nephrologists and nursing personnel was of great importance to encourage the entry and permanency of patients in the program; since the initial interview with the patient, they promoted the importance of doing physical exercise and staying active during the HD session and also outside of the HD center. Although the physiotherapist and the TCAE were those directly involved in the intervention, it was a very positive aspect to convey a unanimous message from all the professionals at the center in favor of the benefits and safety of physical exercise. This was important to motivate patients to carry it out and subsequently establish the program as a regular practice after the study.
The results obtained in our study show how the practice of combined IPE contributes to the improvement of most parameters of physical condition. The increase in meters covered in the 6MWT is associated with an improvement in cardiorespiratory capacity. This increase in cardiovascular function has a positive impact on the physical and functional capacity of people, which exponentially facilitates the performance of basic activities of daily life while reducing the main cause of death in this population. The value obtained in this test has a direct relationship with VO2 max,16 a parameter that is relate with survival. In this sense, other authors have also shown a direct relationship between physical exercise and daily activity with the survival of HD patients as well as the increase in cardiorespiratory capacity. A recent meta-analysis of exercise interventions in HD patients17 confirms that the practice of aerobic and/or combined exercise increases cardiorespiratory capacity regardless of the duration of the program, the intensity of the exercise (not specified in several studies ) and the frequency of execution of the sessions. After a 12-week intervention 30 min of aerobic exercise 3 times/week, a Korean research group18 obtained a statistically significant increase in total distance walked in the 6MWT at the end of the intervention.
Most of the studies use the 6MWT because of the low cost and it is easy to perform.
The value of the mean increase in meters traveled in the 6MWT obtained in our study (47 m) is similar to those found in other publications. Thus, in the meta-analysis published by Clarkson et al.,19 the results of different exercise programs in patients with end-stage kidney disease and dialysis are presented. The 6MWT value increased by 33.6 m with a 95% confidence interval (23.7–43.5); the group that performed IPE increased 36.1 m (23.8–48, 4, 95% CI), regardless of the type of exercise (aerobic or strength). Likewise, the multicenter EXCITE study, with 296 patients, published by Torino et al.20 and Manfredini et al.,21 showed that a low intensity home physical exercise program for 6 months increased the 6MWT by 39 m (33–46, 95% CI) compared to the control group and the increase was 41 m (31–51, 95% CI) n patients who completed 100% of the program. Likewise, another study22 showed that after a 24-week intervention in which patients were randomly assigned to a progressive resistance work or to low intensity aerobic work the mean increase in the value of the 6MWT was 48 m and 20 m, respectively.
Regarding muscle strength, the patients in our study improved both upper and lower body strength. They increased the capacity to grasp using HG and, and significantly improved the strength of extension of the quadriceps in both legs as assessed by dynamometry. Also increased the functional strength of the lower extremities measured with the 10ST; there was a statistically significant reduction in time (seconds) of execution of the test.
In the study by Esteve et al.,23 with a combined 12-week program, aimed at patients over 80 years of age, the mean increase in total kg in HG of was 1.6, the same result as in our study.
Segura-Ortí et al.24 performed a 6-month program of 30 min, isotonic and isometric exercises of the lower limbs during the 3 days/week of HD. The patients had to perform 3 series of 15 repetitions with an intensity between 11 and 15 in the scale of perceived effort. The 10STS result was statistically significant with a mean reduction of almost 5 s, in the test. The small discrepancies with respect to our study may be due to the exclusivity in the type of work (only muscular strength) and in the frequency of exercises (3 days/week).
To determine whether the increase in muscle strength was associated to an increase in muscle mass, the bioimpedance data were analyzed and contrary to the expectations it was observed, a trend towards a decrease in the lean mass index. These results could be due to the fact that the proposed exercise regimen was not intense enough or with the necessary load to obtain changes in muscle structure, although it did achieve improvement in function. To increase lean mass, we believe that the frequency and intensity of strength work needs to be increased, which would achieve peripheral adaptations in the musculoskeletal system and not only central adaptations. That is, not only to improve the synchrony between motor units and a greater recruitment of them, but also to achieve an increase in the size of the muscle fibers.25
The hypothesis was performing of a combined IPE program would contribute to a decrease in BMI and FMI, however, the final results were an increase of almost 1 kg in body weight resulting in an increase in BMI (p = 0.022) and an increase of 1 kg/m2 of the FMI, although it is not reflected a large change in body composition since the variations were very small. These findings could be attributed to the characteristics of our HD population, which tend to increase appetite after starting treatment.26 Also the duration of our IPE program could have been insufficient. These results have also been obtained in other studies where the intervention was exclusively aerobic work. For example, in a French pilot study27 patients performed 30 min of aerobic work intradialysis and the anthropometric changes obtained were similar to the results of our study; after a 3-month intervention, the intervention group did not change the body composition, with a very slight tendency to a reduction in lean mass and an increase in BMI. Also, in another study,28 after 6 months of 30 min pedal 3 days/week at moderate intensity, it was obtained an increase of almost 2 kg/m2 in the value of the FMI.
The results of our intervention are in broad agreement with those obtained in several published studies, with similar interventions, population, pathology and treatments. Although our study shows that there is a trend of improvement in some of physical parameters of our patients, the differences obtained in the different tests are less than the minimum detectable change (MDC) reported in the Segura-Ortí study. and Martínez-Olmos29 for the same tests in the same population. (MDC90 10STS = 8.4 s; MDC 90 6MWT = 66.3 m; MDC 90 HG = 3.4 kg). Therefore, we cannot affirm conclusively that the implementation of IPE is the only cause of this trend.
The fact of not having obtained statistically significant changes in the variations of the pre-post medication or in the dose of HD suggests that the improvements obtained in terms of physical condition could be due to the intervention with IPE. Regarding the results of the analytical parameters, we could not attribute our findings to the IPE intervention itself. Although we suggest that these changes may be due to optimal nutritional monitoring of patients.
Regarding the safety of practicing the IPE program, it was evaluated a potential effect of performing exercise on hemodynamic stability through vascular refilling rate.30 No differences were detected between the vascular filling during the second hour of treatment while performing the intervention, in a situation of IPE and not IPE. This result could be considered a safety indicator for the patient since his hemodynamic status is not significantly modified. Likewise, no significant differences were observed either in blood pressure or HR.
Furthermore a relevant aspect in terms of program security is that no incident was recorded during the IPE session. In 100% of the cases, it was shown that carrying out a physical exercise regimen during dialysis was safe since there were no complications, neither during nor after the completion of its execution. The IPE session was never stopped due to patient discomfort or problems with the AVF. It was only stopped on one occasion due to catheter dysfunction and in no case in patients with AVF. Nor did the blood flow have to be decreased, and there were no hypotensive episodes.
In relation to previous publications, our study contributes an important aspect regarding the dose of exercise, since statistically significant changes have been obtained with 2 weekly sessions, one cardiovascular training and one of resistance strength. It should be noted that with a small stimulus, of frequency and intensity, it has been obtained significant improvements on cardiorespiratory capacity and muscle strength.
Another novel aspect has been the use of vascular filling as a safety parameter of the IPE session. The characteristics of the training have allowed the participant to execute it without observing differences with respect to the day on which the patient did not perform IPE. In addition having set up a good work team that transmitted a unique and positive message towards physical exercise, in reference to the benefits and the safety,has been crucial for its development and execution as a regular practice, fact that will facilitate its continuity in our center.
This study provides positive data, however we are aware that there are some limitations in the present study: the absence of a control group and the limited size of the study sample. It was difficulty to recruit 34 patients, so, to avoid a delay the intervention, it was decided to compare the data obtained in these patients. The frailty and comorbidity of HD patients make it difficult to maintain long interventions without interruptions.
Another great difficulty has been to guarantee the intensity of execution of the exercises. While it is true that the Borg scale is a widely used and a validated subjective method, it does not reach the precision of objective methods. Despite the fact that the intensity of the exercises was increased throughout the intervention (increasing the aerobic work up to 30 min without interruption and adding material in the strength work), there were no objective methods to assess it, as it would have been a percentage of maximum HR or a load in kg closer to the percentage of the maximum repetition required. It would have been interesting to accurately quantify this intensity in order to describe the most correct prescription for these patients based on the desired objectives. Another option would have been to use the modified Borg scale (values from 0 to 10) to facilitate its understanding and to further adjust the effort performed.
ConclusionThe results of our study indicate that the practice of IPE, 2 weekly sessions (one training of each subject matter), contributes to the improvement of cardiorespiratory capacity and upper and lower body muscle strength in HD patients. Furthermore, it has been possible to demonstrate that this practice is safe. It was innovative to analyze this interventions no only with intradialysis events but also with the vascular filling profile. This is a benefit for the patient, since it does not negatively influence the hemodynamic status, as for the course of the HD session.
It has been crucial for the development of the study, and for the constant implementation of the IPE in our center, to have a multidisciplinary team that will be in charge of carrying it out as well as individualizing the load and ensuring the effectiveness of the program.
Key concepts- -
Intradialysis physical exercise contributes to the improvement of cardiorespiratory capacity and muscle strength (both upper and lower extremities) of hemodialysis patients.
- -
This practice is safe both for patients and for the course of the hemodialysis session.
- -
A multidisciplinary team is key to be able to implement this type of program and achieve its continuity within the hemodialysis centers.
This study has not had any source of funding.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Yuguero-Ortiz A, Gomez M, Arias-Guillén M, Ojeda R, Fontseré N, Rodas L, et al. Eficacia y seguridad de un programa de ejercicio físico intradiálisis. Nefrologia. 2021;41:556–565.