Antecedentes: La certificación de biobancos según la norma ISO 9001:2008 pretende mejorar la gestión de los procesos realizados en estos con dos objetivos: la satisfacción del cliente y la mejora continua. En este trabajo se presenta el impacto de la certificación ISO 9001:2008 sobre los procesos de cesión de muestras de un biobanco español especializado en muestras de pacientes renales y con un gran aumento del número de estas entre los años 2009 (12 582 viales) y 2010 (37 042 viales). Métodos: El biobanco de la Red de Investigación Renal española (REDinREN) situado en la Universidad de Alcalá ha puesto en marcha la norma ISO 9001:2008 para la gestión eficaz del material humano cedido a los centros de investigación. Se han analizado mediante encuestas dos períodos en el proceso «cesión de muestras». Durante el primer período, entre las fechas 1-10-12 y 26-11-12 (8 semanas), se han realizado cambios mínimos para corregir errores puntuales. En el segundo período, entre las fechas 7-01-13 y 18-02-13 (6 semanas), se han realizado acciones correctivas generales. Resultados: La identificación de inconvenientes y la puesta en marcha de acciones correctivas para la certificación permitieron: reducir el 70 % del tiempo de ejecución del proceso, aumentar significativamente (200 %) el número de muestras procesadas y mejorar un 25 % el proceso. El aumento del número de muestras procesadas estuvo directamente relacionado con la mejora del proceso. Conclusión: La certificación de la norma ISO 9001:2008, obtenida en julio de 2013, permitió la mejora de los procesos del biobanco REDinREN, aumentando la calidad y la satisfacción del cliente.
Background: Biobank certification ISO 9001:2008 aims to improve the management of processes performed. This has two objectives: customer satisfaction and continuous improvement. This paper presents the impact of certification ISO 9001:2008 on the sample transfer process in a Spanish biobank specialising in kidney patient samples. The biobank experienced a large increase in the number of samples between 2009 (12,582 vials) and 2010 (37,042 vials). Methods: The biobank of the Spanish Renal Research Network (REDinREN), located at the University of Alcalá, has implemented ISO standard 9001:2008 for the effective management of human material given to research centres. Using surveys, we analysed two periods in the “sample transfer” process. During the first period between 1-10-12 and 26-11-12 (8 weeks), minimal changes were made to correct isolated errors. In the second period, between 7-01-13 and 18-02-13 (6 weeks), we carried out general corrective actions. Results: The identification of problems and implementation of corrective actions for certification allowed: a 70% reduction in the process execution time, a significant increase (200%) in the number of samples processed and a 25% improvement in the process. The increase in the number of samples processed was directly related to process improvement. Conclusion: The certification of ISO standard 9001:2008, obtained in July 2013, allowed an improvement of the REDinREN biobank processes to be achieved, which increased quality and customer satisfaction.
INTRODUCTION
Biobanks are indispensable for advancing knowledge about disease and progression markers and for discovering new drugs1. The purpose of biobanks is biomedical research: non-profit public or private establishments that have one or several collections of biological samples of human origin with the aim of biomedical research, organised as technical units with criteria of quality, order and purpose, independently of whether they include samples for other purposes (in accordance with Royal Decree 1716/20112). Therefore, the bases that guarantee the biobank’s success include the implementation of a quality system1,3,4. Within this context, applying generic quality standards allows us to achieve continuous improvement over time.
The generic quality standards most used currently include the ISO 9001:2008 international standard, which is applied to quality management systems (QMS)5. This standard is used to implement and improve the effectiveness of a QMS, increasing customer satisfaction by meeting their requirements. The ISO 9001 is applied by more than one million organisations in 178 countries, with 47% belonging to the European Union. In this regard, Spain was fourth worldwide for ISO 9001 certifications in 20106. Likewise, many biobanks are certified with ISO 9001, such as the UK Biobank (United Kingdom), the UK DNA Banking Network (United Kingdom), Invidumed (Hamburg), the Norwegian Mother and Child Cohort Study Biobank, the Biobank of Picardy, the Graz Biobank (Austria) and the Singapore Biobank1. In Spain, there are biobanks such as DNA and HIV, the biobank of the Hospital Ramón y Cajal, the Basque research biobank and the Diabetes and Associated Metabolic Diseases Network Biomedical Research Centre biobank, amongst others.
Although there is no international standard for the technical quality of these centres, in the good practices guidelines for biobanks published by the Organisation for Economic Cooperation and Development7, the National Cancer Institute8 and the International Society for Biological and Environmental Repositories9, the use of other standards such as ISO 17025 (monitoring and calibration of laboratories)10 and ISO Guide 34 (material production)11 is advised. Moreover, Betsu et al., analysing the proposed standards, recommend using ISO 9001 as a general standard applicable to all biobanks and ISO 17025 and ISO Guide 34 according to the biobank’s activity12.
Biobanks that have decided to improve their processes by following the model presented by ISO 9001 usually certify the management of the processes that they carry out3,13,14. The process-based approach, characteristic of ISO 9001:2008, principally establishes two objectives: customer satisfaction and continuous improvement. As such and bearing in mind the recommendations, when ISO standard 9001 is implemented, the first step is to identify and analyse the biobank’s primary processes. The process monitoring is carried out using quantifiable quality indicators, monitoring activities and by promoting corrective actions (CA). Apart from monitoring, process activities are measured that are key to achieving continuous improvement. These measurements are key elements of QMS15.
In this study, we present the improvement in the transfer process, one of the main processes of a biobank specialised in biological samples, which in our case are blood and urine from chronic kidney disease patients. The substantial increase in samples observed between 2009, with 12,582 vials, and 2010, with 37,042 vials, made it necessary to implement regulation ISO 9001:2008 for the effective management of human material transferred to research centres16. As a result of the improvement, a reduction in process execution time was obtained, accompanied by a significant increase in sample processing. Lastly, the analysis of results allows us to conclude that the increase in the number of samples processed is directly related to the improvement of the process.
METHODS
The biobank that belongs to the Spanish Renal Research Network (REDinREN) is located at the Universidad de Alcalá, in the city of Alcalá de Henares (Madrid), and is financed by FEDER funds, RD6/0016/0002. It specialises in collecting biological samples from patients with kidney disease and it has been operational since 2007.
Development to continually improve the main biobank process
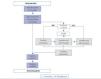
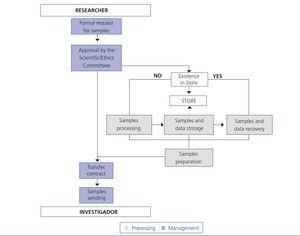
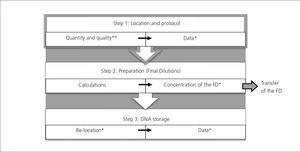
In this section we describe the initial steps and CA that have been incorporated to improve one of the main processes of the REDinREN biobank, based at Alcalá de Henares. The objective of the main biobank process is to transfer DNA samples to REDinREN research centres and affiliated centres and it is very important that the quantity and purity of the samples be maintained. The implementation of the “sample transfer” process has two main parts: in the first part or “management” we took into account the aspects of sample request and management. In the second part or “processing”, we added the tasks that are directly related to the processing of stored blood cell samples from which DNA is isolated (Figure 1). Management includes: the formal request for samples (in accordance with Law 14/2007 on Biomedical Research17), approval by the scientific and ethics committees and signing of the transfer and management contract for the sample sent. Processing includes: isolating DNA samples, which is carried out using an automated robotic nucleic acid extractor, storage, recovering samples and data and lastly, preparing the samples to send out.
The application of this new process was divided into two periods, in each of which the researcher chosen was different, and to carry out the process activities, we did not change the technical teams or management. In the first period, which we called period 1, we carried out initial minimal changes to correct isolated errors, and this period lasted eight weeks. In the second period, which we called period 2, we carried out general CA and this period lasted six weeks.
Process evaluation methods
There were two basic ways of measuring the process quality18: internal measurement (in which we were able to evaluate, amongst others, the increased productivity)19 and the external measurement, which is related to the effects of the market (in which the customer evaluates the quality of the product, amongst other aspects). In our case, we carried out an internal measurement of the process with the aim of evaluating the improvement in productivity. The measurement instrument employed was the “survey”, which was applied using an internal audit form completed by the head of quality. This survey was carried out on management once per period and was composed of 9 questions, and on processing once a week and was composed of 15 questions.
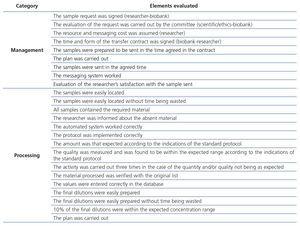
The score was 0 when we identified errors without correction, 0.5 when we identified errors that were corrected and 1 when no errors were identified. We calculated the mean and it was compared with a process in which all elements were 1. As such, we obtained a relative score. The items evaluated are recorded in Table 1.
Statistical analysis
To complete the results analysis, we carried out an analysis of variance (ANOVA) of the quantity of samples and of the processing score. This study tests the hypothesis according to which the means of various samples are equal. In our case, we verified whether there were differences in the quantity of samples processed between the two periods and in the processing score or whether they were the same. For this purpose, we formulated the hypotheses:
H0 samples = There are no differences in the quantity of samples between the two periods.
H0 score = There are no differences in the processing score between the periods.
H1 = The null hypothesis is not true.
We used a significance level of 0.05 for type I errors. To reject the null hypotheses, we had to verify the ratio of the variations between phases and within phases (called F). This value had to be far from its critical value.
We also used the simple linear correlation to establish the relationship between two quantitative variables.
RESULTS
Period 1: start-up
The measurements carried out during the first process application period obtained results that indicated low productivity. The percentage of samples successfully processed was around 25% (44 out of 178). In this first phase, we identified, analysed and summarised a group of problems indicated in the first column of Table 2.
With the application of the CA (Table 2), we made a new process plan in which we highlighted the most significant changes: developing a form, which allowed the exact location of the samples to be obtained; the initial waste of time was thus avoided. The acquisition of a service that provides equipment to replace the automated robotic nucleic acid extractor in the event of breakdown or mechanical failure: this avoided slowing down the activity or manual work. The change in the dissolving of the sample: this allowed us to obtain greater sample productivity, speed and a decrease in identification errors. Last but not least was the segregation of roles: this avoided staff fatigue- and boredom-related errors, and it corrected the lack of data and a lot of isolated errors.
Period 2: verification and application of corrective actions
In the previous section, we mentioned the CA taken to improve the process efficiency. The main objectives in modifying the process were, on the one hand, saving time and processing a greater quantity of samples than in period 1 and, on the other, avoiding fatigue of technical staff. This was achieved while maintaining the quantity and purity of the sample.
After the first period, we saw that, the “better we verify”, the quicker errors are corrected. Therefore, with the aim of carrying out an effective verification, we proposed a series of control points at the most critical steps of the process. As we can observe in Figure 2, step 1 of the sample transfer process (time in which samples are located and the isolation protocol is carried out) has two control points: one at the start and the other at the end. It is only possible to advance from left to right (within one step) if the control points are passed. The same applies for advancing from step 1 to step 2 (from top to bottom).
If a control point is not passed, a planned CA is carried out, another verification is performed and when it is passed, we can advance. A summary of the planned CA for each control point is as follows:
- Quantity and quality: if a pure sample is not obtained and if the concentration is not sufficient. By pure sample we mean that which after processing has 90% of one single intracellular content (for example: DNA, RNA or proteins). By sufficient concentration we mean the sample that, after processing, has an intracellular content quantity that corresponds to the number of initial cells as is indicated in the standard protocol (for example: if we started with 4 million cells, we would expect to obtain 40ng/ul of DNA). CA: perform another isolation with another vial from the same patient, only whenever the quantity and quality have been verified three times beforehand.
- Data: if the data entered in the biobank’s database are not correct. CA: modification of erroneous data by one person, who must always verify the original data printed.
- Final dilution concentration: if when checking 10% of the samples, some do not coincide with the expected concentration. CA: exclude this dilution and carry out the final dilution again; if it is necessary and after verification by the supervisor, quantify the initial sample again. Verify all final dilutions.
- Re-location of vials: if whenever the vials are stored in their corresponding locations they do not coincide. CA: verify the position of all vials to find the error; the vial stored must always be in the original position, in which the database indicates its concentration, quality and volume.
- Data of the sample stored: if the final data added to the biobank’s database are not correct. CA: modification of erroneous data by one person, always verifying the original printed data.
The changes implemented reduce time and improve the process, increasing productivity
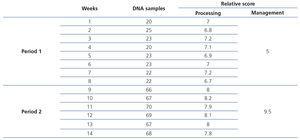
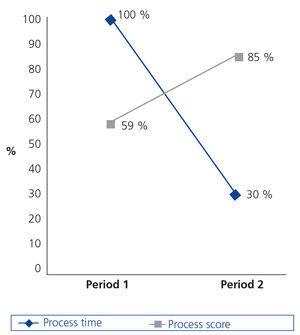
Below, we present the data analysis results (Table 3) obtained by using the process with the changes implemented in period 2. Firstly (Figure 3), we highlight the 70% reduction in time designated to the process. Secondly, we observed an increase in the overall process score, with there being a 25% improvement in our process. The total process score was calculated by adding the contributions of management and processing in each phase.
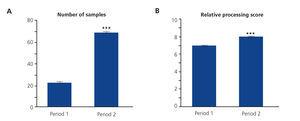
For a more detailed analysis and to find out if we really did have increased productivity by introducing changes in period 2, we carried out an analysis on the quantity of samples processed per week. In Figure 4A, we observed how the number of samples in period 2 increased by 200% with respect to period 1 (from 22.25 to 67.83). On examining the mean processing scores (Figure 4B), we observed a very subtle increase in period 2 (from 6.9 to 8).
This leads us to believe that increased productivity did not depend on the elements evaluated for scoring processing. As such, we carried out a correlation to find out whether they were related and we observed in Figure 5 that there is a clear linear correlation with a coefficient of determination of 0.909. With this analysis, we confirm that the number of samples processed each week is related to the relative processing score.
The one-way analysis of variance (Table 4) to compare variations in the number of samples and in the scoring between the two periods evaluated showed significant variation, both in terms of the quantity of samples (p=4.57 E-18) and in terms of the processing score (p=9.17 E-08).
DISCUSSION
In the main biobank process, in which samples are transferred to REDinRED research centres and affiliated centres, we followed the steps indicated by ISO standard 9001:2008 in the process-based approach. With efficient verification, we were able to implement CA (already documented and not improvised), which led to an increase in productivity. In this study, we quantitatively demonstrated the significant improvement that occurs after the implementation of this standard. We consider that this demonstration of its correct application is of great interest, not only for all REDinREN users, but for all those who are potentially interested in using the samples stored by the biobank in the basic and clinical nephrology research areas. Although the implementation of this standard has previously been reported in haemodialysis units20, this study, for the first time, presents an analysis of processes after the application of this standard. It also reports information from a biobank with exclusive dedication to biological samples of kidney disease patients from all over Spain.
First of all, we obtained an improved process time, saving 70%. Secondly, we obtained an improvement in the process in terms of quantity of samples processed by time. We demonstrated a 200% increase in the processing of samples, which is indicative of a close relationship with the inclusion of improvements in the process, specifically in processing. After carrying out a statistical study, we demonstrated that between the two periods, there were highly significant differences, which increased both the processing score and the sample quantity. Furthermore, we demonstrated that improved processing caused an increase in samples processed, indicating improved productivity in the biobank. We should highlight that the improved productivity in this study was accompanied by better organisation of the technical staff and efficiency of work, the accreditation of the biobank and its registration in the National Spanish Registry of Biobanks at the end of 2013.
In conclusion, the implementation of a generic standard, such as ISO 9001:2008 standard, has improved the productivity of the REDinREN biobank. This guarantees the improvement of our services and the satisfaction of the scientific community, both at a national and international level.
ACKNOWLEDGEMENTS
Carlos III Institute of Health: REDinREN, Spanish Renal Research Network of the Carlos III Institute of Health, FEDER funds, RD12/0021/0006, RD06/0016/0002.
Intensification of Research Activity Programme (ISCIII/Laín-Entralgo Agency/CM).
Íñigo Álvarez Renal Foundation of Toledo (FRIAT)
Reina Sofía Institute of Renal Research (IRSIN).
Vice-dean of Research at the Universidad de Alcalá: Dr. María Luisa Marina Alegre.
Legal advisor of the Research Results Dissemination Office: Ms. Ángela Fuentes Rodríguez.
General Secretary of the Universidad de Alcalá: Dr. Miguel Rodríguez Blanco.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Description of the evaluation elements
Table 2. Summary of problems and corrective actions in period 1
Table 3. Summary of the data evaluated
Table 4. One-way ANOVA results for null hypotheses
Figure 1. Sample transfer process diagram.
Figure 2. Critical phases of the sample transfer process.
Figure 3. Time and score of the main biobank process carried out in two periods.
Figure 4. Comparison of the number of samples and the process score.
Figure 5. Linear correlation between the number of samples and the processing score.