Análisis de la vida media del injerto y de su tasa de pérdida en 1045 transplantes de donantes cadáver adultos entre 1986 y 2001, con seguimiento hasta 2011, clasificados en dos periodos en función de la inmunosupresión: 1986-1995 y 1996-2001. La curva de Kaplan-Meier mostró un aumento significativo de la supervivencia del injerto durante el periodo 1996-2001. La vida media real no censurada del injerto fue de 10,25 años en 1986-1995 y la actuarial fue de 14,58 años en 1996-2001 (p < 0,001). La tasa de pérdida del injerto fue significativamente mayor en 1986-1995, incluso con la exclusión del primer año del análisis. En 1996-2001, la disminución de la función renal fue menos pronunciada, observándose una mejor conservación a pesar de que los donantes tenían más edad y de que habían fallecido por accidente cardiovascular. El modelo parsimonioso multivariante de Cox reveló que la edad del donante, el rechazo agudo, el panel de anticuerpos reactivos, el tiempo de isquemia fría y la función retrasada del injerto se asociaban de forma significativa a un mayor riesgo de pérdida del injerto. Sin embargo, el riesgo de pérdida del injerto se vio reducido en un 21% en 1996-2001 en comparación con el periodo 1986-1995. Se observó una reducción similar (25%) al incluir el tratamiento con MMF en el modelo multivariante en lugar del periodo de estudio. La supervivencia del injerto a largo plazo mejoró significativamente en 1996-2001 frente al periodo 1986-1995, a pesar de que los donantes tenían más edad. Por lo tanto, la inmunosupresión moderna podría haber contribuido a la mejora de los resultados del transplante renal.
We analyzed graft half-life and attrition rates in 1045 adult deceased donor kidney transplants from 1986-2001, with follow-up to 2011, grouped in two periods (1986-95 vs. 1996-01) according to immunosuppression. The Kaplan-Meier curve showed a significant increase in graft survival during 1996-2001. The uncensored real graft half-life was 10.25 years in 1986-95 and the actuarial was 14.58 years in 1996-2001 (P<0.001). The attrition rates showed a significantly greater graft loss in 1986-95, even excluding the first year from the analysis. The decline in renal function was significantly less pronounced in 1996-2001, indicating better preservation of renal function, despite the increase in donor age and stroke as the cause of donor death. The parsimonious Cox multivariate model showed donor age, acute rejection, panel reactive antibody, cold ischemia time and delayed graft function were significantly associated with a higher risk of graft loss. In contrast, the risk of graft loss fell by 21% in 1996-2001 compared with 1986-95. A similar reduction (25%) was observed when MMF treatment was entered into the multivariate model instead of study period. Long-term graft survival improved significantly in 1996-2001 compared to 1986-1995 despite older donor age. Modern immunosuppression could have contributed to the improved kidney transplant outcome.
INTRODUCTION
Since the introduction of cyclosporine (CsA) controversy has surrounded the long-term results of renal transplantation (RT). The most accepted theory is derived from an observational study of kidney transplant recipients who received their grafts between 1988 and 1995; this study showed a significant increase in graft survival during the first year, but only a marginal increase in the long term.1 These data demonstrated that, despite CsA reducing the incidence of acute rejection (AR), RT remained a limited option in terms of survival, with the continued loss of grafts.
Other single center2 and multicenter3,4 studies including transplant recipients who received their grafts after 1995 and whose immunosuppression included the new agents mycophenolate mofetil (MMF) and tacrolimus (TaC) found an increased long-term graft survival, or at least showed a significant improvement in the rate of decline in long-term kidney allograft function in recent years.5 In any case, long-term graft loss remained an important problem in kidney transplant recipients, due mainly to calcineurin inhibitor (CNI) toxicity-mediated allograft damage.6
Later, other authors provided data showing that graft loss due to nephrotoxicity was less usual than previously considered, though it was nevertheless common after antibody-mediated rejection, glomerulonephritis and nonadherence to the treatment.7,8 This represented a new approach to the causes of graft loss, and obliged us to determine whether the introduction of the potent new immunosuppressive agents MMF and TaC improved the results of RT in terms of survival. The aim, therefore, of this study was to assess whether the long-term RT outcome has improved under modern immunosuppression in a cohort study derived from a single transplant center. Given that important immunosuppressive changes occurred in 1996, we arbitrarily divided our cohort population into two periods (1986-1995 and 1996-2001) in order to perform a more detailed and real analysis of factors associated with RT outcome.
MATERIALS AND METHODS
Study design
This retrospective cohort study was carried out at a single transplant center. We pooled data from all adult patients (>18 years) who received a primary or repeat deceased donor transplantation from January 1986 to December 2001 at Carlos Haya University Hospital (Malaga, Spain), with a follow-up to December 2011. Thus, the minimum and maximum follow-up times were 10 years and 25 years, respectively. Only 17 (1.6%) patients had an incomplete follow-up during the whole study period. Patients with a living donor or double transplant (combined kidney-pancreas or kidney-liver) were excluded.
The endpoint of the study was to analyze the graft survival and the attrition rates stratified by year of transplantation in recipients who received different immunosuppressive regimens. We also assessed the patient survival rate and the evolution of renal allograft function.
Immunosuppression
Table 1 shows the immunosuppressive treatment in our cohort population by study period: 1986-95 versus 1996-01. Most patients in the first period received CsA plus prednisone (P). Thereafter, MMF was added in the majority of immunosuppressive treatments, as well as TaC instead of CsA with effect from 1999.
Clinical variables
The study variables included cause of donor death (trauma or stroke), age and gender of the donor and the recipient, body mass index, first or retransplantation, time on dialysis, panel-reactive antibody (PRA) at peak and at transplantation, HLA mismatches, cold ischemia time (CIT), the presence of delayed graft function (DGF), AR, type of immunosuppressive therapy, graft function, graft and patient survival, graft half-life, and graft attrition rates.
Attrition rates
To calculate the attrition rates, we first obtained the actual 0-1 year, 1-3 year, 3-5 year and 5-10 year survival rates. We then subtracted the number of patients with graft failure during the time period from the original number of patients in the cohort. The resulting number was then divided by the original number in the cohort to obtain an absolute failure percentage. This percentage of absolute failures was divided by the total number of years in the follow-up interval, with which we obtained the yearly failure rates.4
Renal function
The estimated glomerular filtration rate (GFR: mL/min./1.73m2), based on the serum creatinine concentration, was obtained using the abbreviated Modification of Diet in Renal Disease (aMDRD) equation9 at three months and annually.
Institutional review and patient protection
Medical record review was performed according to the Spanish law on clinical data confidentiality. This study was approved by the ethics committee of the hospital and was conducted according to the principles described in the Declaration of Helsinki.
Statistical analyses
A descriptive analysis was made, obtaining the mean and the standard deviation for the quantitative variables and the relative frequency for the qualitative variables. Hypothesis contrast tests used included the Student t test or Mann-Whitney test for quantitative variables and the Χ2 test or Fisher test for qualitative variables.
Kaplan-Meier survival curves were used to assess the unadjusted half-lives and survival rates for both death-censored and uncensored graft survival. The graft half-life was calculated as the median survival, i.e., the cut point or intersection on the Kaplan-Meier curve with the 50% threshold. The graft half-life was considered to be real if all the patients reached this point and actuarial if only some reached this point. The log-rank test was used to compare survival curves.
The Cox proportional regression model was used to estimate the risk factors for graft loss during the follow-up period, with backward stepwise regression, i.e., starting with all the possible variables and interactions that could influence the model. The variables were then eliminated if their presence failed to improve the quality of the model, choosing always the most parsimonious model, which finally included just those variables giving a more precise prediction.
Covariates used to calculate the adjusted Cox multivariate proportional hazards models included donor and recipient age, AR, CIT, DGF, PRA, cause of donor death, HLA mismatches, period of RT (1996-01 vs. 1986-95) and immunosuppression (CsA, MMF, TaC and induction with poly- and monoclonal antibodies). The proportionality of the exploratory variables in the model was assessed by visual inspection of the log-log survival plots.
The statistical analyses were done with SPSS (IBM, Chicago, IL, version 15) for Windows (Microsoft, Redmond, WA), and EPIDAT 3.1 (Programa para analisis epidemiologico de datos tabulados; Xunta de Galicia, Santiago de Compostela, Spain), and significance was set at P<0.05.
RESULTS
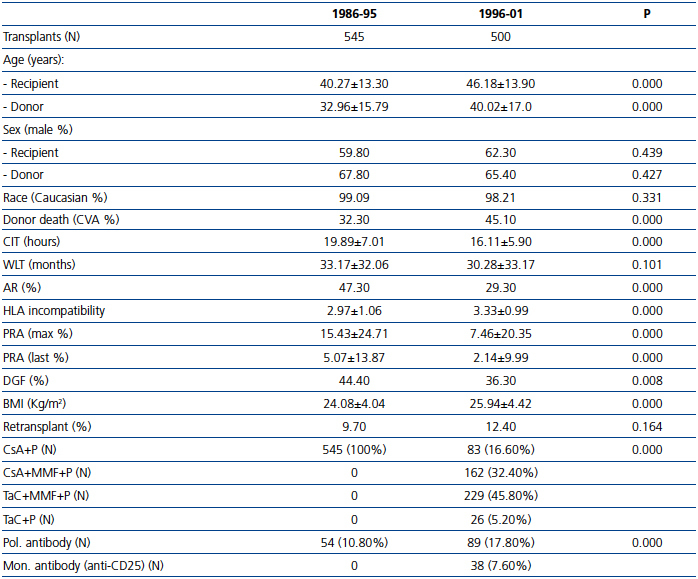
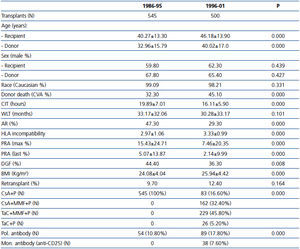
We analyzed a total of 1045 adult cadaveric donor kidney transplant recipients between 1986 and 2001. Most of the patient and donor demographic and clinical characteristics varied between the two study periods (Table 1). Significant increases were found in donor and recipient age, donor death from stroke, and number of HLA mismatches in the second period. In contrast, a significant reduction was seen in PRA, incidence of AR, and CIT in the second period.
Graft survival
The global actuarial graft half-life was 11.5 years. The real graft half-life in patients with AR was 7.7 years and in those without AR the actuarial graft half-life was 15 years (P<0.001).
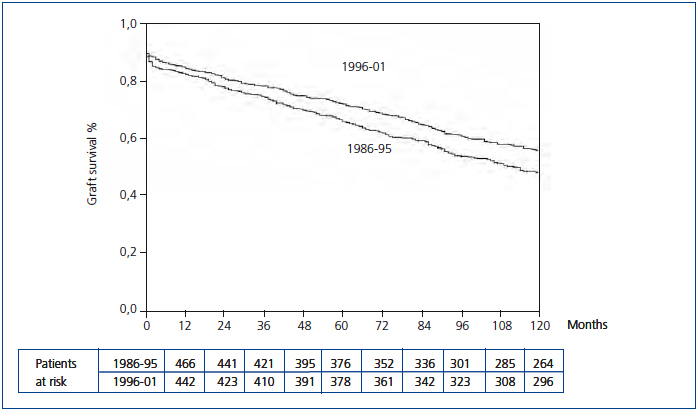
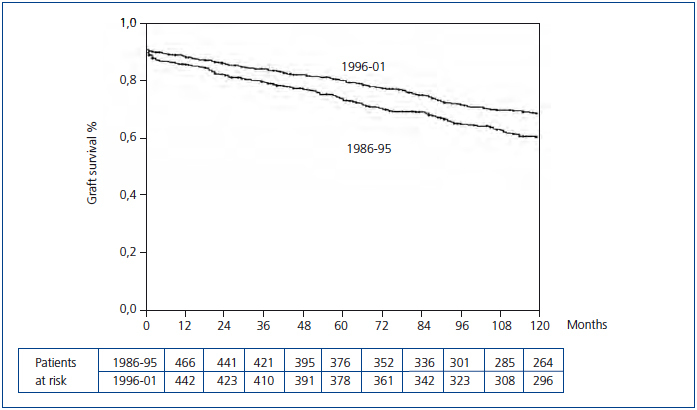
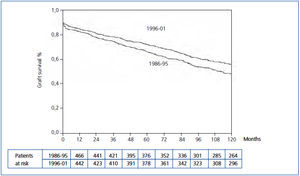
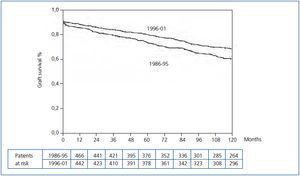
The Kaplan-Meier curve showed a significant increase in uncensored and death-censored graft survival during the second period (Figure 1 and Figure 2). The real uncensored graft half-life was 10.25 years in the first period and the actuarial graft half-life was 14.58 years in the second period (P<0.001). For the censored cases, the actuarial graft half-life was 14.83 years in the first period, whereas at this point in those patients transplanted during the second period 61.3% still had a functioning graft.
Graft attrition rate %
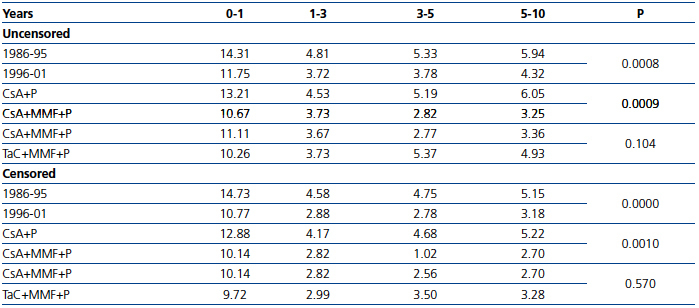
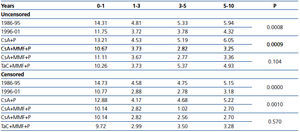
The attrition rate %, censored and uncensored, showed a continuous loss of grafts over time in both periods, significantly greater in the first period (Table 2). These differences remained after excluding the first year from the analysis (P=0.0018 and P=0.0001, respectively). Similarly, patients who received CsA+MMF+P showed a significantly lower rate of graft loss compared with patients on CsA+P in both the uncensored (P=0.0009) and censored (P=0.001) cases. No significant differences were seen in graft survival between CsA and TaC.
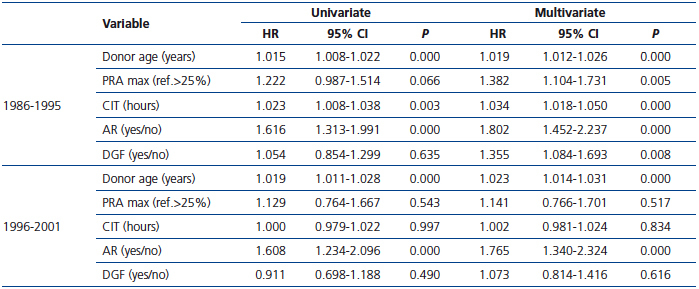
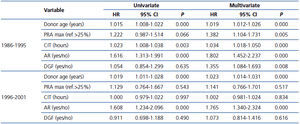
The Cox univariate and multivariate models showed that donor age and AR were the only risk factors that were significantly associated with graft loss in both periods of the study (Table 3).
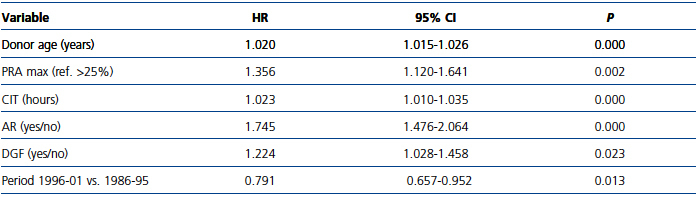
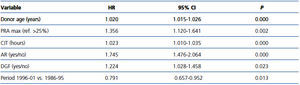
The parsimonious Cox multivariate model showed that donor age, AR, PRA, CIT and DGF were significantly associated with a higher risk for graft loss (Table 4). In contrast, the risk of graft loss fell by 21% in the period 1996-01 compared with the period 1986-95 (Table 4). Given that most patients (72.20%) transplanted during the period 1996-01 received MMF, we entered into the final model MMF treatment instead of study period. Notably, the use of MMF was associated with a similar reduction in the risk for graft loss (HR 0.758, 95% CI 0.623-0.924; P=0.006), whereas neither the use of CsA vs. TaC nor induction with poly- and monoclonal antibodies showed significant differences in graft survival.
Patient survival
No significant differences were found in patient survival between the two study periods. The 10-year patient survival was 83.2% in the first period and 83.3% in the second period (P=0.432), even though the recipient age was significantly higher in the latter period.
Renal function
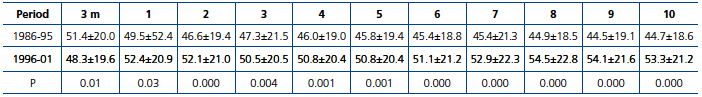
The GFR was significantly better in the second period with effect from the third month post-transplant. At this third month post-transplant, a higher GFR was observed in patients transplanted in 1986-95 compared with 1996-01 (51.4±20.0 vs. 48.3±19.6mL/min./1.73m2; P=0.01). However, with effect from the first year post-transplant the renal function was significantly better in the second study period, despite a higher donor age in this period (Table 5).
DISCUSSION
For years the concept has prevailed that graft survival after kidney transplantation has increased in the first year but not over the long term. That is, progress had been made during the early phase of RT, a stage in which T-cell mediated rejection and acute kidney injury predominate,8 but problems still existed over the longer term that limited the half-life of the graft. Among the causes of this failure, CNI nephrotoxicity was considered a determining factor.6 This suggestion was supported by authors who found chronic lesions in the native kidneys of recipients of other organs (not kidney) who were treated with CNI.10
Nowadays, though, whilst still recognizing the nephrotoxic effect of CNI, it is not universally accepted that the chronic lesions attributed to CNI are caused solely by these drugs.11-13 After analyzing the causes of long-term graft loss, others have shown that in cases with fibrosis/atrophy a specific cause was identified in 81% and it was rarely attributable to CNI toxicity alone,14 cell mediated rejection, or acute kidney injury.8 Graft loss was, however, more common after antibody-mediated rejection or glomerulonephritis and 47% of cases of graft loss were identified as being due to nonadherence to treatment.8
These data reflect changes in the reasons limiting graft half-life after RT, with a tendency towards inadequate control of the immune response (particularly humoral) over the long term, either due to the limited effect of the immunosuppression used or to lack of treatment adherence.
Taking these data into consideration, we analyzed what has happened over the last 25 years at our transplant center, starting with the hypothesis that the efficacy of the immunosuppression used is determinant in the long-term results of RT. Indeed, a higher graft survival rate was observed in patients who received a kidney transplant in the second study period (1996-01), with modern immunosuppression using MMF and TaC. In addition, when MMF was entered instead of study period into our final multivariate model, the use of MMF was associated with a 25% reduction in the risk of graft loss. As a consequence, this has resulted in an increase in uncensored graft half-life of 10.25 years at 14.58 years in those treated with MMF (P<0.001). These data show a significant change in the long-term results of cadaveric RT in our setting. Furthermore, this effect was independent of the presence of AR and other classical risk factors for graft loss such as donor age, PRA, CIT or DGF, which supports the beneficial effect in terms of graft survival of modern immunosuppression with MMF. Nonetheless, we cannot rule out the potential beneficial effect for graft survival of other factors introduced during the study period 1996-01, such as the increased use of cardioprotective and renoprotective drugs during this period (data not shown). Further longitudinal studies are needed to clarify this issue.
The initial conclusions from the database of the United States Renal Data System on the effect of MMF on graft survival were published in 2000. The study compared, retrospectively, the graft survival of two cohorts of recipients treated with CsA+P plus MMF or Aza (azathioprine). The conclusion was that censored graft survival at four years was significantly greater in those treated with MMF, with effect from both the time of transplant (85.6% vs. 81.9%, P<0.0001) and from six months after RT (91.4% vs. 89.8%, P=0.002). The multivariate analysis showed that AR conferred a relative risk 2.41 times greater of developing what, at that time, was defined as chronic allograft nephropathy, whereas the absence of AR reduced the risk by 19% in those treated with MMF (RR=0.81, P<0.001). In addition, treatment with MMF was a protective factor for graft survival, independently of its preventive effect on AR, and for long-term patient survival.15 These data were confirmed in a study undertaken before ours2 as well as in the present study, which provides longer-term follow-up data and shows the continued reduction in graft loss during the whole post-transplant period in those patients who received MMF in association with a CNI.
In patients with biopsy-confirmed interstitial fibrosis and tubular atrophy, the addition of MMF reduced progression of worsening renal function, independently of plasma CsA levels.16 MMF treatment has also been associated with substantially reduced fibrosis in the glomerular, microvascular and interstitial compartments,17 all determinant factors in long-term renal function.
These observations suggest that the addition of MMF to a CNI will achieve a better control of the humoral response, the cause currently thought to be the main factor responsible for long-term graft loss.8 Indeed, there is clinical and experimental evidence that treatment with MMF inhibits antibody formation. Kidney transplant patients who received triple-drug therapy with CsA+P+MMF had reduced antibody levels to the influenza vaccine compared with those who received triple-drug therapy with Aza;18 and in patients receiving an ABO-mismatched kidney transplant, the administration of MMF reduced the formation of anti-blood type IgG, which is increased after withdrawal of MMF19 and treatment with MMF was shown to be superior to Aza in maintaining a renal response to treatment and in preventing relapse in patients with lupus nephritis.20 However, no significant differences have been found in the rate of graft loss with the use of CsA or TaC plus MMF.
Analysis of the renal function in the two study periods is of particular interest. With the increase in donor age and stroke as the cause of death, we would expect worse renal function in the second period (1996-01). However, the data show the exact opposite. The decline in renal function was significantly less pronounced in the latter period, indicating better preservation of renal function. This may be related with the better control of the immune response.
This study is not without limitations, especially those inherent to a single-center retrospective cohort study. In addition, we are unaware of the causes of graft loss in some cases as biopsy findings were not always available, though the renal function was recorded in all cases. Furthermore, the use of newer immunosuppressive agents such as mTor inhibitors was not included as the follow-up period would have been too short. Nevertheless, the study included a rigorous follow-up, with extremely few patients lost to follow-up. Finally, although this study did not include a competitive risk model, survival analyses in large populations over a long period of time (as in this study) guarantee the reliability of the results.21
In conclusion, long-term graft survival improved significantly in the period 1996-2001 as compared with 1986-1995 despite the older age of the donors. Modern immunosuppression, including MMF, has contributed to optimizing RT outcome.
ACKNOWLEDGMENTS
The authors thank the Nephrology and Urology Unit teams from Carlos Haya University Hospital (Malaga, Spain) for their collaboration. This study was partly supported by the Spanish Ministry of Economy and Competitiveness (MINECO) (Grant no. PI10/01020) from the Instituto de Salud Carlos III, RETICS (REDINREN RD12/0021/0015), and by Grant PI-0590-2012 (in part) from the Consejería de Salud del Gobierno de Andalucía. We also thank Ian Johnstone for his linguistic assistance in the preparation of the text.
Conflict of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Demographic data of the donors and recipients of cadaveric kidney transplants in 1986-2001, separated into two periods according to the study period, with the type of immunosuppression regimen used
Table 2. Comparative data for graft loss (attrition rate %) stratified by years post-transplant, for different groups and immunosuppression
Table 4. Parsimonious Cox proportional hazards multivariate model of factors impacting on graft survival
Table 5. Course of renal function (mL/min/1.73m2) in transplant patients in the periods 1986-95 and 1996-01
Figure 1. Kaplan-Meier uncensored graft survival according to transplant period (Log-rank test, P=0.000)
Figure 2. Kaplan-Meier censored graft survival according to transplant period (Log-rank test, P=0.000)
Table 3. Cox analysis of risk factors for graft loss according to time period