Tumor necrosis factor-α inhibitors (TNF-α inhibitors) are potent immunomodulators and have been associated with the development of autoimmunity, such as glomerulonephritis.1,2 Recently, investigators described a case of IgA nephropathy in patients with inflammatory bowel disease on a prolonged treatment with TNF-α inhibitors in sustained clinical remission of their intestinal disease, and with improvement after discontinuation of the drug, making the case less likely to be an extraintestinal manifestation.3,4
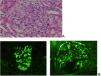
In this regard we e present two clinical cases from our hospital:Case 1 The first case is a 20-year-old male, with Crohn's disease (CD) since 2013, who started treatment with infliximab and azathioprine, went into complete remission, and continued in monotherapy with infliximab since 2016. After seven years on treatment with infliximab (in April 2020), the patient suffered acute deterioration of renal function (Cr 1.8 mg/dL CKDEPI 51 mL/min /1.73 m2), nephrotic proteinuria (8 g/24 h) and microhematuria. A renal biopsy was performed with the finding of IgA nephropathy, M1 E0 S1 T0 in the Oxford classification (Fig. 1). Complete sustained remission of his CD was confirmed, infliximab was discontinued, and an angiotensin-II receptor antagonist was started at maximum tolerated doses and corticosteroids (prednisone 1 mg/kg/day for one month followed by a tapering regimen and discontinuation after six months). After 14 months, his CD relapsed, with no associated deterioration of renal function, and ustekinumab was started, with a good response. After 18 months of renal biopsy, he had normal renal function (Cr 1.1 mg/dL CKDEPI 90 mL/min/1.73 m2), complete improvement of proteinuria (0.3 g/24 h) and no microhematuria. (A) Optical microscopic image showing glomerulus with mesangial proliferation at the expense of cellularity and matrix. (B) Immunofluorescence image with abundant and intense mesangial deposition for IgA (left) and C3 (right), this antibody also localized in Bowman's capsule of some glomeruli. A 51-year-old woman with extensive Crohn's ileitis, stenosing, fistulous and with intra-abdominal abscess on debut in 2012, started treatment with azathioprine and infliximab in 2013 and have been in complete remission ever since. Azathioprine was discontinued in 2017 and infliximab monotherapy was maintained.
In March 2020, acute deterioration of renal function (Cr 1.6 mg/dL CKDEPI 36 mL/min /1.73 m2) together with proteinuria (0.4 g/24 h) and microhematuria was detected.
A renal biopsy was performed which confirmed the diagnosis of IgA nephropathy (M1 E0 S0 T1). Her CD remained in complete remission, infliximab was discontinued, and she received a course of corticosteroids (prednisone 1 mg/kg/day for one month and a tapering regimen with discontinuation of prednisone after six months). After 20 months she remains in complete remission of CD without specific treatment, with improvement in renal function (Cr 1.39 mg/dL CKDEPI 44 mL/min/1.73 m2), remission of proteinuria (0.2 g/24 h) and no microhematuria.
We present these two cases that support the relationship between the prolonged use of infliximab in patients with CD and the development of IgA nephropathy, also observed in other autoimmune pathologies such as rheumatoid arthritis, psoriasis and ankylosing spondylitis.1,2
Although it is a rare complication, it is clinically important, as 25–30% of IgA nephropathy cases develop end-stage renal disease within 20–25 years of diagnosis, and in 10% of patients end-stage renal disease is achieved within five years of diagnosis. Early detection of this renal pathology allows early action to reverse or at least minimize kidney damage. In the case of secondary IgA nephropathy, the main treatment is to treat/eliminate the cause.5
As for the mechanism, it is postulated that the inhibition of TNF-α exerts a direct effect on lymphocyte function and cytokine production, changing the T-helper cytokine response from type 1 to type 2 secreting IL-4, IL-5 and IL-10, promoting humoral immunity, and inducing antibody production. These antibodies cross-react with aberrant IgA1, which upon deposition in the mesangium leads to complement activation and the development of IgA nephropathy.1
Therefore, it is of great importance to alert physicians using TNF-α inhibition to this possible adverse effect, and for them to perform routine analyses of renal function, proteinuria (in 24 h urine or in isolated urine with protein/creatinine ratio or albumin/creatinine ratio) and urine sediment. If there are alterations, they should refer patients to nephrology, as early diagnosis and treatment are essential for renal prognosis.
FundingThis work has not received any funding.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank the participants of the manuscript for their involvement and contribution to research in the field of nephrology.