Resistant hypertension (RH) represents an important multi-organic impact and increases the morbi-mortality. We aimed to evaluate the evolution of hypertensive mediated organ damage in patients with RH after adding spironolactone.
Material and methodsRetrospective study of 58 patients with RH who started spironolactone (12.5–25mg daily). Office blood pressure, 24-h ambulatory blood pressure monitoring (24h-ABPM), urine albumin-to-creatinine ratio and echocardiographic parameters were analyzed prior to initiation of spironolactone and after 12 months of treatment.
ResultsThirty-six percent of patients were women and mean age was 67.3±10.1 years. We observed a decrease in urine albumin-to-creatinine ratio (median [RIQ25–75]) of 27.0 (7.5–255.4) to 11.3 (3.1–37.8)mg/g, p=0.009. This was more relevant in patients with albuminuria grade A2 and A3: 371.2 (139.5–797.4) to 68.4 (26.5–186.5)mg/g, p=0.02. The echocardiographic changes were: posterior wall thickness: −1.0±0.4mm (p<0.001), interventricular septal thickness: −0.6±0.5mm (p=0.01), left ventricular (LV) mass index: −14.7±10.2g/m2 (p=0.006), LV remodeling index: −0.04±0.036 (p=0.03), without statistically significant changes in LV ejection fraction, LV end-diastolic diameter, LV end-systolic diameter, left atrial diameter, relationship between early ventricular filling wave and atrial contraction and LV filling pressure index.
Systolic/diastolic office blood pressure decreased −12.5±4.9/−4.9±3.0mmHg, p<0.001. In 24h-ABPM, systolic and diastolic BP had a significant decrease in diurnal and nocturnal periods and 38.1% of patients presented a favorable change in the circadian pattern, p<0.001.
ConclusionsAdding spironolactone to patients with RH contributes to improve hypertensive mediated organ damage by reducing albuminuria levels and echocardiographic parameters of hypertensive heart disease.
La hipertensión arterial resistente (HTAR) supone un importante impacto a nivel multiorgánico e incrementa la morbimortalidad. Este trabajo evalúa la evolución de la lesión orgánica mediada por hipertensión en pacientes con HTAR tras añadir espironolactona.
Material y métodosEstudio retrospectivo de 58 pacientes con HTAR a quienes se añadió espironolactona (12,5–25mg/día). Se obtuvieron parámetros de presión arterial clínica y MAPA-24h, cociente albúmina/creatinina y datos ecocardiográficos previos a iniciar espironolactona y tras 12 meses de tratamiento.
ResultadosEl 36,2% de los pacientes eran mujeres y la edad media de 67,3±10,1 años. Se objetivó un descenso en albuminuria (mediana [RIC25–75]) de 27,0 (7,5–255,4) a 11,3 (3,1–37,8)mg/g (p=0,009), siendo más marcado en pacientes con albuminuria grado A2 y A3: de 371,2 (139,5–797,4) a 68,4 (26,5–186,5)mg/g, p=0,02.. A nivel ecocardiográfico se evidenció: pared posterior: −1,0±0,4mm (p<0,001), tabique interventricular: −0,6±0,5mm (p=0,01), índice de masa del ventrículo izquierdo (VI): −14,7±10,2 g/m2 (p=0,006), índice de remodelado del VI: −0,04±0,036 (p=0,03), sin cambios estadísticamente significativos en fracción de eyección VI, diámetro diastólico VI, diámetro sistólico VI, diámetro de aurícula izquierda, relación entre onda de llenado ventricular temprano y contracción auricular ni en índice de presión llenado VI.
La presión arterial clínica sistólica/diastólica presentó un descenso de −12,5±4,9/−4,9±3,0mmHg, p<0,001. En los MAPA-24h se observó un descenso significativo de presión arterial sistólica y diastólica en los períodos diurno y nocturno, y un cambio favorable en el patrón circadiano en el 38,1% de los pacientes, p<0,001.
ConclusionesAñadir espironolactona en HTAR contribuye a la reducción de la lesión orgánica mediada por hipertensión a nivel de albuminuria y de parámetros ecocardiográficos de cardiopatía hipertensiva.
Resistant hypertension (RHTN) is a major public health problem for primary care doctors and specialists today. Its prevalence is estimated to be between 10%–20% of the treated hypertensive population1–3 and these patients have a higher risk of experiencing major cardiovascular events causing morbidity and mortality.3,4
The Clinical Practice Guidelines of the European Society of Hypertension and the European Society of Cardiology recommend using spironolactone, an aldosterone receptor antagonist, as the fourth drug in patients with RHTN.2 The antihypertensive efficacy of spironolactone in patients with RHTN has been widely confirmed in the control of clinical blood pressure (BP), self-measured BP monitoring at home and in 24-h ambulatory blood pressure monitoring (24h ABPM).5–7 However, few studies detail the variation in the circadian pattern of BP following the introduction of spironolactone. In addition, spironolactone has a beneficial effect on the reduction of myocardial fibrosis and ventricular remodelling in patients with heart failure (HF),8–10 and in the reduction of proteinuria in patients with hypertension and pathological albuminuria or diabetic nephropathy.11–13 The scientific evidence for cardiac benefits after spironolactone primarily focuses on HF patients.8–10 In particular, studies in hypertensive patients are limited to reporting a reduction in left ventricular mass.14,15 Studies focusing on patients with RHTN and providing more echocardiographic data are scarce or have smaller samples.16 This study aimed to evaluate the evolution of hypertension-mediated organ damage (HMOD) at the cardiac and renal levels in patients with RHTN after spironolactone was added to antihypertensive therapy.
MethodsPopulationThis was a retrospective observational study of patients diagnosed with RHTN who had spironolactone added to their antihypertensive therapy (starting dose 12.5–25mg/day), seen at the Hypertension and Vascular Risk Unit of the Nephrology Service of Hospital del Mar [del Mar Hospital], Barcelona, from April 2016 to September 2018, with subsequent follow-up until January 2020. The study was approved by the local Institutional Ethics Committee in accordance with the Declaration of Helsinki.
The specific objectives were: (i) to evaluate the effect of spironolactone on HMOD, according to echocardiographic parameters and albuminuria, 12 months after its addition to antihypertensive treatment; (ii) to evaluate the efficacy of spironolactone in the control of clinical BP and 24h ABPM, as well as changes in the circadian pattern of BP; (iii) to analyse whether there are associations between changes in BP and changes in HMOD after adding spironolactone.
Patients aged 18 years or older, diagnosed with RHTN, defined according to the guidelines from the European Society of Hypertension3 as BP>140/90mmHg despite receiving treatment with at least three antihypertensive drugs of different therapeutic classes, including a diuretic, were included. The diagnosis of RHTN was confirmed by 24h ABPM (24h BP>130/80mmHg).
Studied variablesClinical and laboratory data were evaluated at baseline and 12 months after the start of spironolactone. Demographic and medical history data were collected, including age, sex, body mass index and abdominal circumference, presence or absence of concomitant pathologies (cardiovascular disease [ischaemic heart disease, cerebrovascular disease, and peripheral vascular disease], type 2 diabetes mellitus [T2DM], dyslipidaemia, chronic kidney disease [CKD], and obstructive sleep apnoea syndrome); and laboratory parameters (estimated glomerular filtration rate [eGFR],17 potassium and glycated haemoglobin [HbA1c] in plasma and urinary albumin excretion [UAE] measured by urine albumin–creatinine ratio), before spironolactone was started and after 12 months of treatment. Albuminuria grades were defined as: A1 as EUA lower than 30mg/g, A2 as EUA 30–300mg/g and A3 as EUA higher than 300mg/g.18
Echocardiographic parametersThe following parameters were analysed by transthoracic echocardiogram validated with 2.0–4.0MHz transducers using a Vivid E9 system (GE Healthcare) in two-dimensional mode and colour Doppler, with the patient supine and non-tilted: posterior wall (PW) thickness, interventricular septal (IVS) thickness, left ventricular ejection fraction (LVEF), left ventricular mass index (LVMI), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter, left ventricular remodelling index (LVRI=2×PW/LVEDD), left atrial diameter, relationship of early ventricular filling wave to atrial contraction (E/A), and left ventricular filling pressure (LVFP) index (E/e’). These determinations were performed at baseline and after approximately 12 months of spironolactone treatment. In addition, left ventricle (LV) geometric patterns were also assessed.
AlbuminuriaAlbuminuria was evaluated by immunonephelometry and expressed by the urine albumin–creatinine ratio determined in first morning urine. Biochemical parameters were obtained with autoanalysers using standard methods. Again, these determinations were performed before starting spironolactone treatment and at 12 months.
Blood pressureClinical BP was determined using a validated semi-automatic device (Omron® 705IT) with a suitably-sized cuff for the brachial circumference of each subject. At each visit, three BP measurements spaced 1–2min apart, were performed after five minutes of rest with the patient seated, and the final value was the mean of the three measurements.
BP by 24h ABPM was determined by a validated semi-automatic device (Spacelabs 90207-5Q) with a suitably-sized cuff for each patient. BP monitoring was started between 8am and 10am on a weekday, with systolic BP (SBP) and diastolic BP (DBP) values obtained every 20min during the waking and sleeping periods. These periods were defined according to the times reported by the patients that they woke up and went to sleep on the day of the test. A minimum of 80% valid readings was required to consider the record good quality and to be accepted as valid.
Data were collected on antihypertensive drugs prescribed at baseline and 12 months after starting spironolactone. Treatment adherence was evaluated by self-reported patient data, with a systematic review at each visit.
Statistical analysisStatistical measures of central tendency and arithmetic mean (95% confidence interval [95% CI]) were used for continuous variables and frequency distribution for discrete variables. The median (25th and 75th percentiles) was used for continuous variables that did not follow a normal distribution. The variation of the parameters of interest at 12 months of follow-up was studied using linear mixed models. The Wilcoxon test was used to evaluate the change in albuminuria, a parameter of non-normal distribution. Correlations between the SBP and DBP difference in time and changes in HMOD were evaluated using Pearson’s correlation coefficient.
All analyses were adjusted for age, sex, body mass index, T2DM and estimated glomerular filtration rate. A p value <0.05 was considered statistically significant. The program used for the statistical analysis was STATA 15.1 (StataCorp, CollegeStation, TX, USA).
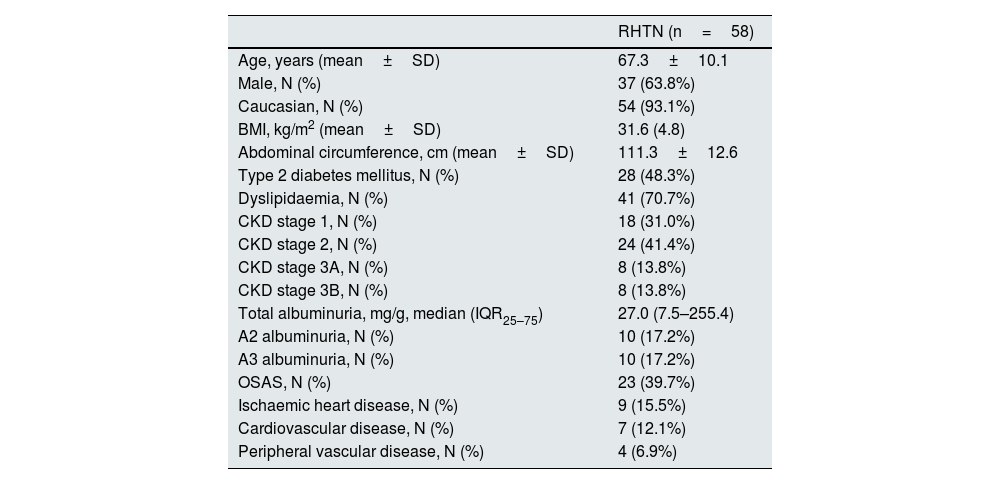
ResultsA total of 58 patients were included, with a mean age of 67 years; the majority were Caucasian males with a high prevalence of dyslipidaemia and obesity (Table 1).
Baseline characteristics of patients with resistant hypertension.
| RHTN (n=58) | |
|---|---|
| Age, years (mean±SD) | 67.3±10.1 |
| Male, N (%) | 37 (63.8%) |
| Caucasian, N (%) | 54 (93.1%) |
| BMI, kg/m2 (mean±SD) | 31.6 (4.8) |
| Abdominal circumference, cm (mean±SD) | 111.3±12.6 |
| Type 2 diabetes mellitus, N (%) | 28 (48.3%) |
| Dyslipidaemia, N (%) | 41 (70.7%) |
| CKD stage 1, N (%) | 18 (31.0%) |
| CKD stage 2, N (%) | 24 (41.4%) |
| CKD stage 3A, N (%) | 8 (13.8%) |
| CKD stage 3B, N (%) | 8 (13.8%) |
| Total albuminuria, mg/g, median (IQR25–75) | 27.0 (7.5–255.4) |
| A2 albuminuria, N (%) | 10 (17.2%) |
| A3 albuminuria, N (%) | 10 (17.2%) |
| OSAS, N (%) | 23 (39.7%) |
| Ischaemic heart disease, N (%) | 9 (15.5%) |
| Cardiovascular disease, N (%) | 7 (12.1%) |
| Peripheral vascular disease, N (%) | 4 (6.9%) |
A2 albuminuria: urinary albumin excretion 30–300mg/g; A3 albuminuria: urinary albumin excretion >300mg/g; BMI: body mass index; CKD: chronic kidney disease; OSAS: obstructive sleep apnoea syndrome; SD: standard deviation.
Before spironolactone was started, patients received an average of 3.8±1.2 antihypertensive drugs: 100% a diuretic (71.3% thiazide and 28.7% loop); 94.2% a renin–angiotensin–aldosterone system blocker (84.5% ARB and 15.5% ACE inhibitor); 87.9% a calcium channel blocker; 60.7% a beta-blocker; 35.4% an alpha-blocker; 10.2% a sympatholytic (clonidine or moxonidine); 3.4% a direct renin inhibitor (aliskiren); and 2.4% an arterial vasodilator (hydralazine).
The initial daily dose of spironolactone (median [IQR 25–75]) was 25mg (12.5–25) and 25mg (12.5–37.5) at 12 months.
Regarding renal safety, a significant potassium elevation of 0.36±0.02mmol/l and a reduction in estimated glomerular filtration rate at −6.0±1.0ml/min/1.73m2 was detected at 12 months of treatment, p<0.05 in both cases.
Changes in hypertension-mediated organ damageAlbuminuria decreased from an initial median (IQR 25–75) of 27.0mg/g (7.5–255.4) to 11.3mg/g (3.1–37.8) at 12 months, p=0.009. In patients who had A2 or A3 albuminuria at baseline, this reduction was especially pronounced: from 371.2mg/g (139.5–797.4) initially, to 68.4mg/g (26.5–186.5) finally, p=0.02. The decrease in albuminuria was not correlated with a weight change in patients (body mass index variation of +0.3kg/m2 after 12 months of follow-up, correlation r=−0.20; p=0.2), nor with HbA1c variation in patients with T2DM (HbA1c variation of −0.05% after 12 months, correlation r=0.14, p=0.5). No patients were diagnosed with new T2DM onset during the study.
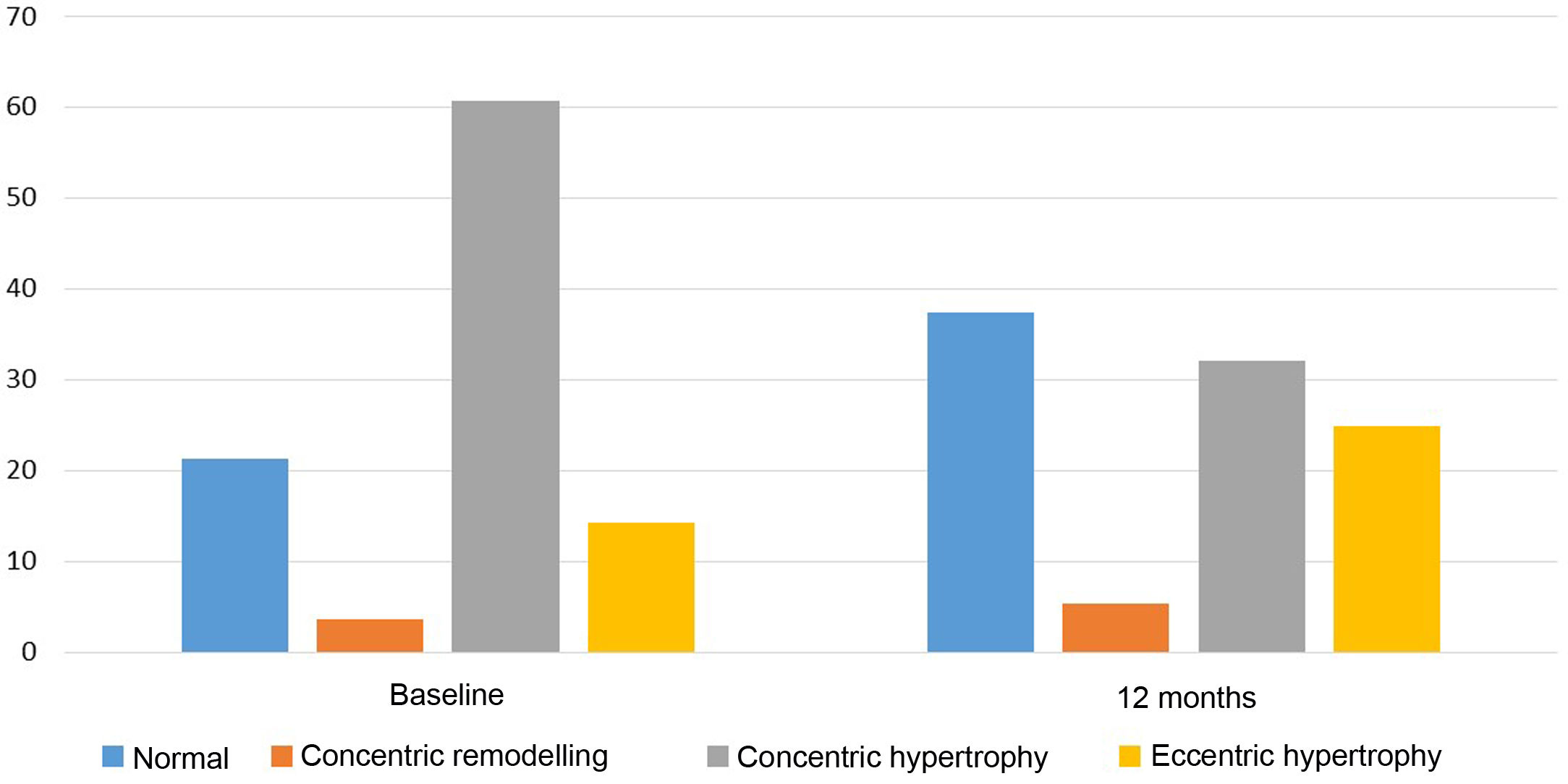
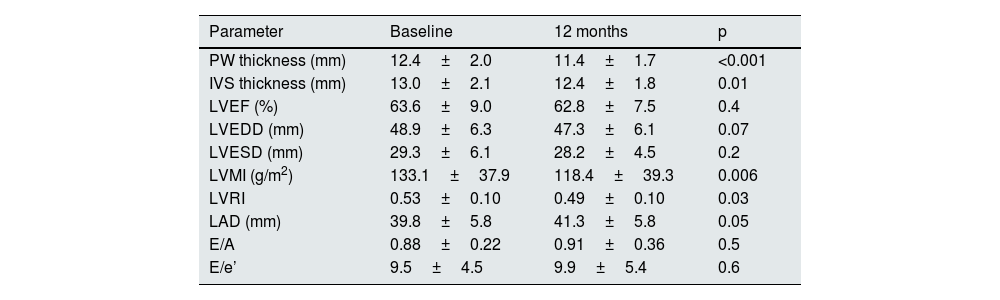
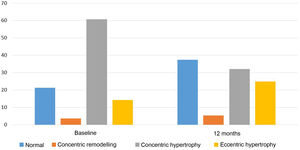
Regarding echocardiographic parameters (Table 2), a reduction in PW thickness (mean [95% CI]) of −1.0mm (−1.4 to −0.6), p<0.001, IVS thickness of −0.6mm (−1.1 to −0.1), p=0.01, LVMI of −14.7g/m2 (−24.9 to −4.4), p=0.006, and LVRI of −0.04 (−0.08 to −0.004), p=0.03 was observed. No statistically significant changes were found in the variation of LVEF, LVEDD, left ventricular end-systolic diameter, left atrial diameter, E/A, or E/e’. In addition, the proportion of patients with pathological LVMI (>95g/m2 in women or >115g/m2 in men) decreased from 72.4% at baseline to 56.9% after 12 months of spironolactone treatment (p<0.001). With regard to LV geometric patterns (Fig. 1), the concentric hypertrophy pattern was initially predominant, present in 60.7% of patients, and prevalence decreased to 32.1% after 12 months of treatment (p<0.001). The echocardiographic normal LV pattern was in the majority at 12 months of spironolactone treatment (37.5% of patients), whereas it was 21.4% before treatment. Overall, 21.4% of patients experienced an improvement in their baseline LV geometric pattern, p=0.001.
Change in echocardiographic parameters after 12 months of spironolactone treatment.
| Parameter | Baseline | 12 months | p |
|---|---|---|---|
| PW thickness (mm) | 12.4±2.0 | 11.4±1.7 | <0.001 |
| IVS thickness (mm) | 13.0±2.1 | 12.4±1.8 | 0.01 |
| LVEF (%) | 63.6±9.0 | 62.8±7.5 | 0.4 |
| LVEDD (mm) | 48.9±6.3 | 47.3±6.1 | 0.07 |
| LVESD (mm) | 29.3±6.1 | 28.2±4.5 | 0.2 |
| LVMI (g/m2) | 133.1±37.9 | 118.4±39.3 | 0.006 |
| LVRI | 0.53±0.10 | 0.49±0.10 | 0.03 |
| LAD (mm) | 39.8±5.8 | 41.3±5.8 | 0.05 |
| E/A | 0.88±0.22 | 0.91±0.36 | 0.5 |
| E/e’ | 9.5±4.5 | 9.9±5.4 | 0.6 |
E/A: relationship of early ventricular filling wave to atrial contraction; E/e’: left ventricular filling pressure index; IVS thickness: interventricular septal thickness; LAD: left atrial diameter; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVMI: left ventricular mass index; LVRI: left ventricular remodelling index; PW thickness: posterior wall thickness.
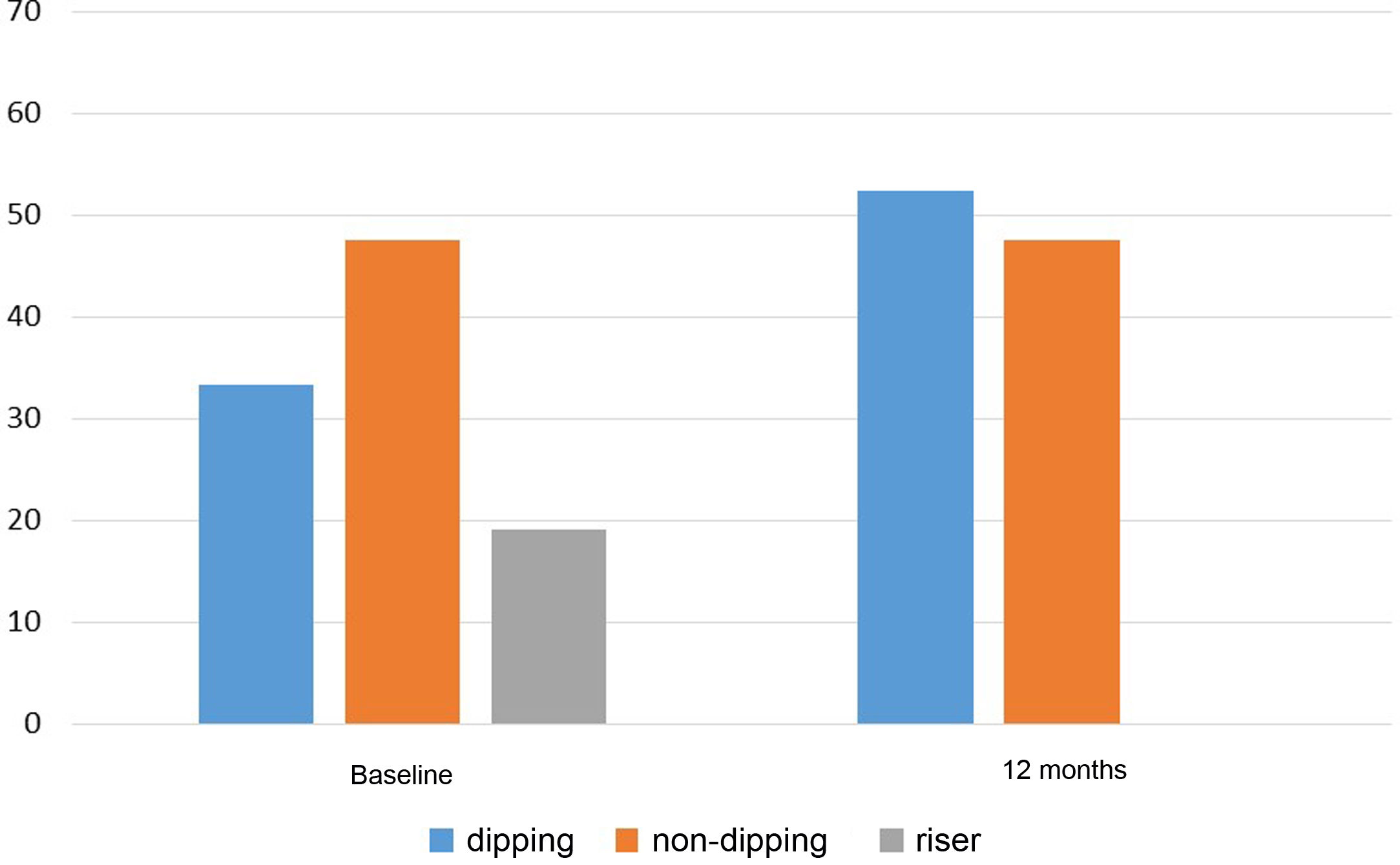
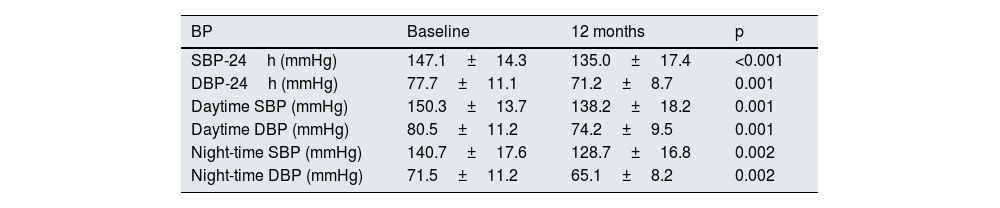
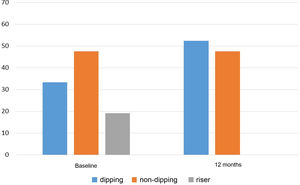
With regard to clinical BP, a significant reduction in SBP from 152.0±16.0mmHg to 139.5±14.4mmHg and DBP from 83.1±13.4mmHg to 78.2±10.7mmHg was observed at 12 months after the addition of spironolactone, p<0.001 in both cases. Sixty-point-four per cent of patients with insufficient control of clinical BP before starting spironolactone achieved adequate control (SBP≤140mmHg and DBP≤90mmHg) after 12 months of treatment. This percentage was higher when the BP changes were analysed according to the 24h ABPM (the initial and final 24h ABPM was available for 36 of the 58 patients). Thus, Table 3 shows the results obtained in the 24h ABPM records, with a decrease in SBP and DBP in both the daytime and night-time periods. Blood pressure normalised in 66.7% of patients (SBP 24h≤130mmHg and DBP 24h≤80mmHg) after 12 months of treatment. Regarding the circadian patterns of the 24h ABPM (Fig. 2), the non-dipping pattern predominated initially, present in 47.6% of patients. After 12 months of treatment, the predominant pattern was the dipping pattern (52.4%), and the riser pattern disappeared, p<0.001. Thirty-eight point one per cent of patients experienced a favourable change in their circadian BP pattern (understood as a change from riser to non-dipping, from riser to dipping, or from non-dipping to dipping), p=0.002.
Blood pressure variation by 24h ABPM after 12 months of spironolactone treatment.
| BP | Baseline | 12 months | p |
|---|---|---|---|
| SBP-24h (mmHg) | 147.1±14.3 | 135.0±17.4 | <0.001 |
| DBP-24h (mmHg) | 77.7±11.1 | 71.2±8.7 | 0.001 |
| Daytime SBP (mmHg) | 150.3±13.7 | 138.2±18.2 | 0.001 |
| Daytime DBP (mmHg) | 80.5±11.2 | 74.2±9.5 | 0.001 |
| Night-time SBP (mmHg) | 140.7±17.6 | 128.7±16.8 | 0.002 |
| Night-time DBP (mmHg) | 71.5±11.2 | 65.1±8.2 | 0.002 |
24h ABPM: 24-h ambulatory blood pressure monitoring; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Four patients (6.9%) initially had A2 or A3 albuminuria, concentric left ventricular hypertrophy and a riser pattern in 24h ABPM. Three of them improved in the three parameters of HMOD after 12 months with spironolactone, and the fourth showed improvement exclusively in the level of albuminuria.
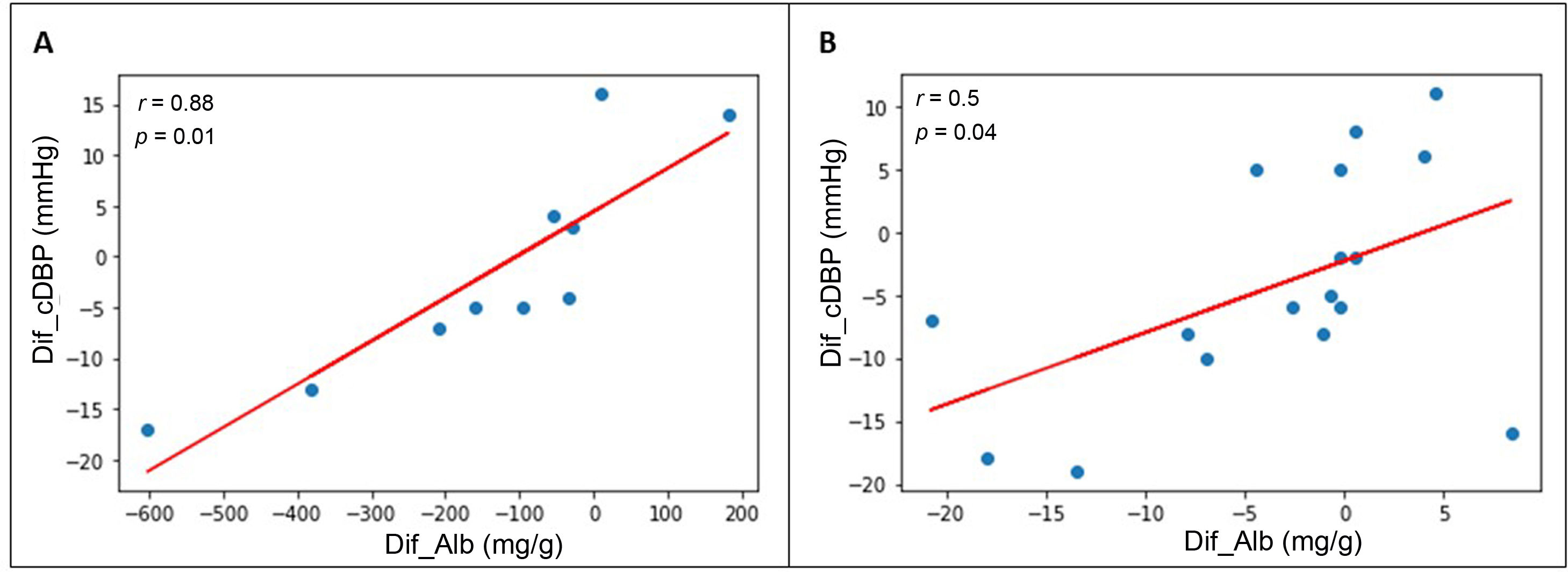
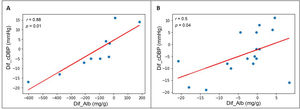
Association between BP changes and HMOD changesThe reduction in albuminuria correlated with the decrease in clinical DBP obtained after the introduction of spironolactone: r=0.46; p=0.02. When the patients with A1 albuminuria and those with A2 or A3 albuminuria were analysed separately (Fig. 3), a correlation with DBP with greater statistical power was found: r=0.50 in the group with baseline A1 albuminuria (p=0.04) and r=0.88 in the group with baseline A2 or A3 albuminuria (p=0.01). We found no correlations between a reduction in albuminuria and a decrease in clinical SBP. Nor did we find correlations between echocardiographic changes (PW thickness, IVS thickness, LVMI, and LVRI) and the decrease in clinical SBP or DBP. In patients with A2 or A3 albuminuria at baseline, the reduction in albuminuria correlated with a reduction in PW thickness after the introduction of spironolactone (r=0.40; p=0.04), but no correlation was found between reduced albuminuria and changes in IVS thickness, LVMI, or LVRI.
Correlation between reduction in albuminuria and reduction in DBP after 12 months of spironolactone treatment in patients with initial A2 or A3 albuminuria (A) and initial A1 albuminuria (B).
DBP: diastolic blood pressure; Dif_Alb: difference between final and initial albuminuria; Dif_cDBP: difference between final and initial clinical DBP.
The main finding of this study is the confirmation that spironolactone use in patients with RHTN reduces HMOD at the cardiac and renal levels. The echocardiographic benefits have been reproduced in the literature mainly in patients with HF,8,10 although the data are more limited in patients without HF. Edwards et al.14 observed a reduction in left ventricular mass in patients with CKD, and Pitt et al.15 also demonstrated a reduction in left ventricular hypertrophy in hypertensive patients with eplerenone 200mg/day, but studies focusing on patients with RHTN treated with spironolactone are scarce. Gaddam et al.16 observed a reduction in left ventricular mass, IVS thickness, and LVEDD in patients with RHTN after six months of treatment with 25–50mg/day of spironolactone, although the cohort analysed was of a reduced sample size (n=11) and follow up was six months. All previous studies focused on reducing the percentage of patients with left ventricular hypertrophy, without providing other data regarding changes in echocardiographic parameters. In contrast, our study analyses the changes in each echocardiographic parameter. In a novel way, the different LV geometric patterns and the changes experienced after spironolactone treatment are shown (thus, 21.4% of patients showed an improvement concerning the initial LV geometric pattern).
With regard to the antiproteinuric action, the beneficial effect of spironolactone is similar to that observed mainly in patients with diabetes or CKD and hypertension.11–13 Our study found an improvement in albuminuria after 12 months of spironolactone treatment in patients with RHTN, in whom the prevalence of T2DM and CKD were 48% and 27%, respectively, which is lower than in the studies reported. We ruled out that the improvement in albuminuria was related to weight loss in patients during the study or a significant improvement in HbA1c in diabetic patients. In addition, we found a correlation between the decrease in DBP achieved after introducing spironolactone and the reduction in albuminuria, suggesting that the antiproteinuric effect of spironolactone should be related to antihypertensive efficacy. On the contrary, the improvement in echocardiographic parameters did not correlate with the decrease in BP, so it could be an intrinsic effect of the drug itself or occur through other mechanisms not analysed here, such as changes in the renin–angiotensin–aldosterone axis. These data would be consistent with previously published information on the cardiological and renal benefits obtained from using angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers,19,20 which are drugs that also interfere with the renin–angiotensin–aldosterone system.
In all of the studies mentioned above, the reduction of HMOD was achieved with an initial dose of spironolactone of 25–50mg/day and a subsequent dose escalation to 50–100mg/day. In our study, the initial dose of spironolactone was 12.5–25mg/day and the final median dose was 25mg/day, with the cardiovascular and renal protective benefits of spironolactone being achieved at doses that appeared to be lower than in previous studies.
The antihypertensive efficacy of spironolactone observed in this study is comparable to that of previous studies.5–7 In relation to the 24h ABPM records, a reduction of SBP and DBP in both periods, daytime and night-time, has been reported6,7 previously, but few studies detail how the circadian pattern of BP varies after spironolactone is added. Our study provides data on these changes, with the conversion of 100% of patients with a riser pattern to circadian profiles with lower cardiovascular risk after 12 months of treatment, as well as a significant improvement in the circadian pattern according to the initial 24h ABPM in 38.1% of patients. These findings contribute to an improvement in the cardiovascular risk profile of these patients.
The study’s main limitations include the fact that this is a retrospective, single-centre observational study. In addition, the sample is limited by the requirement for an initial echocardiogram and another echocardiogram after 12 months of spironolactone treatment, which is not always performed in all patients at our centre for whom this drug is added. Therefore, fewer patients than those receiving spironolactone in actual clinical practice could be analysed.
In conclusion, spironolactone is a drug that is effective in the reduction of HMOD, that is, hypertensive heart disease and albuminuria, as well as in the improvement of the circadian pattern of BP in patients with RHTN, which contributes to the improvement of the cardiovascular risk profile of these patients.
Key concepts- •
In RHTN, adding spironolactone reduces HMOD.
- •
Spironolactone is effective in reducing hypertensive heart disease and albuminuria.
- •
The use of spironolactone was related to an improvement in the 24h ABPM circadian pattern.
This study has received partial funding from the ISCIII [Instituto de Salud Carlos III (San Carlos III Institute)] project — RETICS [Redes Temáticas de Investigación Cooperativa en Salud (Thematic Networks for Cooperative Health Research)] Sub-programme, and RETICS and REDinREN [Renal Research Network] funds RD16/0009/0013.
Conflicts of interestThe authors declare that they have no potential conflicts of interest related to the content of this article.