Antecedentes: Hipotéticamente, la utilización de dosis altas de antagonistas del receptor AT1 de angiotensina II, al bloquear más el receptor AT1, debería producir mayores beneficios que el uso de de dosis convencionales. Objetivo: Evaluar los efectos sobre proteinuria y función renal con dosis ultraaltas de irbesartán en la nefropatía diabética establecida. Material y método: Estudio prospectivo de intervención no controlado ni aleatorizado de 3 años de seguimiento, utilizando un tratamiento multifactorial basado en 600 mg diarios de irbesartán. Se analizan variables demográficas, antropométricas y analíticas al inicio y final del estudio. Se incluyeron 40 pacientes (75% con diabetes tipo 2) con promedio de edad de 57,1 ± 10 años, 29 (72,5%) hombres, con índice de masa corporal (IMC) de 30,7 ± 5 kg/m2. Resultados: La presión arterial sistólica (157,6 ± 27 vs. 130,1 ± 14) y diastólica (88,8 ± 10 vs. 76,2 ± 8 mmHg) se redujeron significativamente (p < 0,001) al final del estudio. El perfil lipídico mejoró significativamente. La kaliemia no se modificó. La creatinina sólo aumentó 0,17 mg/dl, aunque fue significativo (p < 0,05), y el filtrado glomerular estimado se redujo (69,8 ± 29,7 vs. 60,25 ± 23,0 ml/min/m2) (p < 0,05). La proteinuria se redujo de 2,4 ± 1,99 a 0,98 ± 1,18 g/24 h (p < 0,001). La reducción promedio fue 59,2%, y el 25% de los pacientes se hizo normoalbuminúrico. Salvo IMC y hemoglobina glucosilada, todos los objetivos recomendados por la American Diabetes Association se alcanzaron. Ningún paciente abandonó el estudio por efectos secundarios. Conclusión: El tratamiento de la nefropatía diabética establecida con dosis ultraaltas de irbesartán se mostró muy eficaz y seguro en reducir la proteinuria y retardar la progresión hacia la insuficiencia renal crónica terminal.
Background: Hypothetically, the greater the blockade of angiotensin AT1 receptors from ultra-high doses of angiotensin receptors blockers (ARB), the greater the expected renoprotection effects. The aim of our study was to evaluate the effects of ultra-high doses of irbesartan on proteinuria and renal function in diabetics with established or overt diabetic nephropathy (ODN). Material and Method: Ours was a prospective, non-randomised 3-year follow-up study, using a multifactorial therapeutic approach based on irbesartan 600mg daily. Demographic variables, anthropometric data, and biochemical parameters were comparatively analysed at the beginning and end of the study. Forty patients (75% with type 2 diabetes) were included, average age 57.1±10, 29 male (72.5%). Results: SBP (157.6±27mm Hg vs 130.1±14mm Hg) and DBP (88.8±10mm Hg vs 76.2±8mm Hg) decreased significantly at the end of follow-up (P<.001). Serum creatinine increased by only 0.17mg/dl, although this was a statistically significant difference (P<.05). Proteinuria markedly decreased from 2.64±1.99 to 0.98±1.18 (P<.0001), i.e. 59.2%. Twenty-five percent of patients had normal albuminuria at the end of the follow-up period. Lipid profiles significantly improved. No patients withdrew from the study due to side effects, and serum potassium did not change significantly over the course of the study. Except for BMI and HbA1c, all other therapeutic targets set out by ADA recommendations improved significantly. Conclusions: The treatment of ODN with ultra-high doses of irbesartan was highly effective and safe in reducing proteinuria and slowing the progressive course to ESRD.
INTRODUCTION
Currently, established or overt diabetic nephropathy (ODN) is the primary cause for starting dialysis in Spain, with very high associated cardiovascular mortality and morbidity rates.1,2 As a result, all therapeutic strategies that aid in slowing the progression towards advanced chronic kidney disease (ACKD) and the appearance of cardiovascular complications are welcome.
As established by current nephrology guidelines,3,4 the approach to diabetic patients with chronic kidney disease (CKD) must be multifactorial, with well-established objectives aimed at effectively reducing blood pressure (BP) and proteinuria, controlling other associated vascular risk factors, and pharmacologically blocking the renin-angiotensin system (RAS). This multifactorial approach allowed us to significantly reduce macro/microangiopathic complications in diabetes.5
Considering the important role that the intra-renal activation of the RAS plays in the pathophysiology of ODN,6 finding an effective method for blocking this pathway is very important to slow down the progression to advanced or terminal CKD.
Several therapeutic alternatives based on the pharmacological blockade of the RAS are used in primary, secondary, and tertiary prevention of ODN.7 These alternatives include the use of angiotensin II receptor (AT1) blockers (ARB).8,9 Although these studies demonstrated the renoprotective benefits of this drug as compared to placebos and other medicines such as amlodipine, this treatment poses a persistently high residual risk of renal function deterioration in these patients, and approximately 30% of patients under treatment suffer a mid-term progression towards ACKD.10
ARB have a dosage-dependent inhibitory effect on angiotensin II.11 However, the doses recommended for clinical practice, which perhaps were established taking into account the direct relationship between increased dose and decreased tolerability of angiotensin-converting enzyme (ACE) inhibitors, have been based on their effectiveness as anti-hypertensive drugs, which involves a flat dose-response curve following upward titration. However, titration of ACE inhibitors or ARB based solely upon their anti-hypertensive efficacy is inadequate for blocking renal tissue RAS satisfactorily.11,12
As such, at least hypothetically, if the pathophysiological effect of angiotensin II (allowing its entrance into cells) is based on the AT1 receptor, the higher the percentage of AT1 receptors that are blocked, the higher the renoprotective benefit will be.13
With this in mind, the aim of our study was to perform a long-term evaluation of a multifactorial treatment based on irbesartan 600mg/day in ODN patients and its effects on proteinuria, renal function, and the level of compliance with the therapeutic targets set out by the American Diabetes Association (ADA).3
MATERIAL AND METHOD
Ours was a prospective, non-randomised study with a multifactorial approach and no controls, including patients fulfilling the clinical criteria for diagnosing ODN.4 In 4 patients (10%), the diagnosis was made based on a biopsy. We excluded patients with non-diabetic CKD, proteinuria <300mg/day, serum creatinine (sCr) ≥4mg/dl, severe infectious or neoplastic disease before or during the study, chronic liver disease, pregnant women, estimated survival of less than 3 years, or patient refusal to participate.
All patients were informed as to the study objectives and provided informed consent for participation. After inclusion in the study, patients were started on a multifactorial treatment regimen designed to achieve the therapeutic targets proposed by the ADA.3 The treatment was based on irbesartan 600mg/day (300mg in the morning and 300mg in the afternoon), which was chosen for this study based on previous evidence of its benefits in treating ODN.9
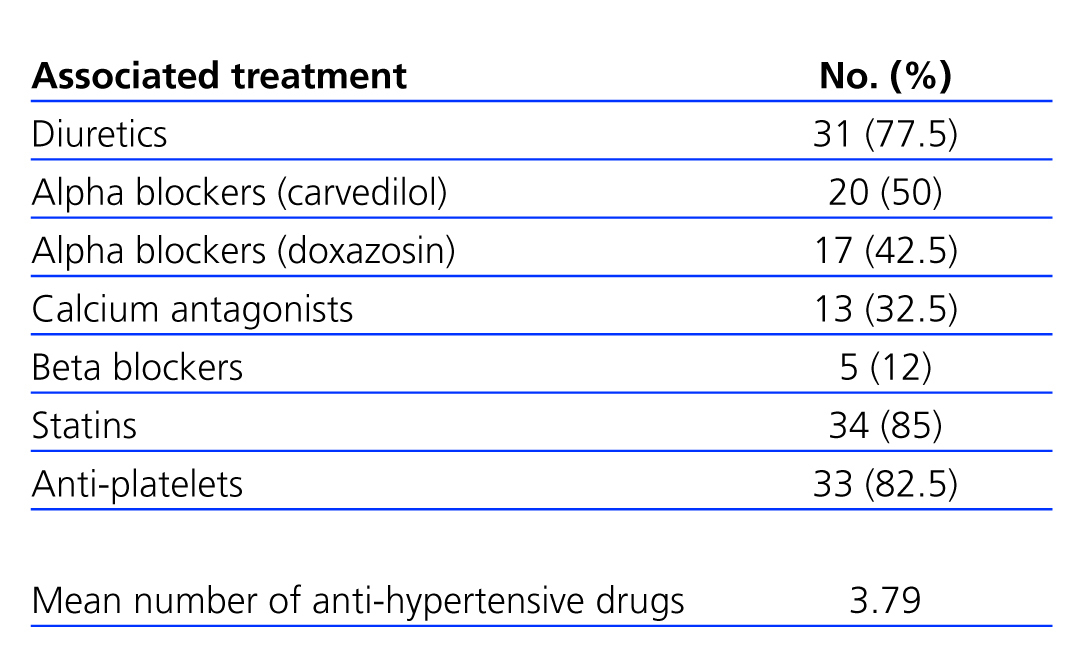
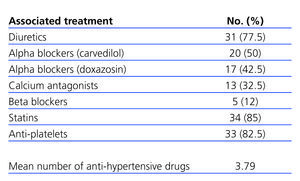
All patients were recommended to adopt standard hygienic/dietary measures aimed at reducing vascular risk,14,15 along with a moderate restriction in protein intake (0.6-0.8g/kg/day).4 The anti-hypertensive treatment was supplemented with other drugs (Table 1) with the objective of maintaining BP<130/80mm Hg, and when possible <125/75mm Hg,14,16 with a mean 4 anti-hypertensive drugs (including irbesartan) administered per patient.
All patients were monitored every six months until completing the 3-year follow-up period.
We compiled demographic variables (age and sex), time from diagnosis of diabetes mellitus and arterial hypertension, type of diagnosis (clinical/histological), type of diabetes (1 or 2), prescription of insulin or oral anti-diabetics, statins and anti-platelets, anthropometric variables (body mass index [BMI], waist circumference, systolic blood pressure [SBP] and diastolic blood pressure [DBP] following the protocols established by the European Society,2 pulse pressure [PP] in mm Hg and heart rate [HR]), associated cardiovascular risk factors, and micro/macroangiopathic complications. Renal function was estimated using sCr (in mg/dl using the modified Jaffe method) and glomerular filtration rate (GFR) using the abbreviated MDRD formula [186.3 x sCr-1.154 x age-0.203 x (0.742 for women) x (1.21 for African Americans)]. We used 24-hour urine samples to determine: proteinuria (grams), sodium concentration (mmol), and urea concentration (g/l, as an indirect indicator of protein intake). We also measured kalemia (mEq/l), baseline glycaemia (mg/dl), glycosylated haemoglobin (HbA1c, %), haemogram, lipid profile, C-reactive protein (CRP), and uric acid (mg/dl).
All blood samples were extracted in the central testing laboratory between 8.00 am and 10.00 am, on an empty stomach.
Statistical analysis
Qualitative variables were expressed as mean + standard deviation (SD), and categorical variables were expressed as percentages and 95% confidence intervals (95% CI). We used Student’s t-tests to compare means for numerical variables between the two phases of the study period (baseline and final). We used Fisher’s exact test to compare categorical variables and Spearman’s rank correlation coefficient to examine the relationship between different quantitative variables. Three different variables were considered for the analysis of proteinuria: baseline proteinuria, proteinuria after treatment, and percent reduction in proteinuria. None of the three variables had a normal distribution, and so non-parametric tests for this statistical analysis were used. Even so, the sample size was larger than 30, and so we also applied parametric tests. The results for both tests were consistent. We also performed a detailed descriptive analysis of these three variables to test for normality, calculating asymmetry and kurtosis coefficients, and the Shapiro Wilk test was used to test for normal distribution. We compared the results from before and after treatment using the Wilcoxon test for paired samples. Creatinine clearance (initial and final) did have a normal distribution, and so we used a paired Student’s t-test for the analysis of this variable. We considered a P-value <.05 to be statistically significant. All analyses were performed using SPSS software, version 12.0.
RESULTS
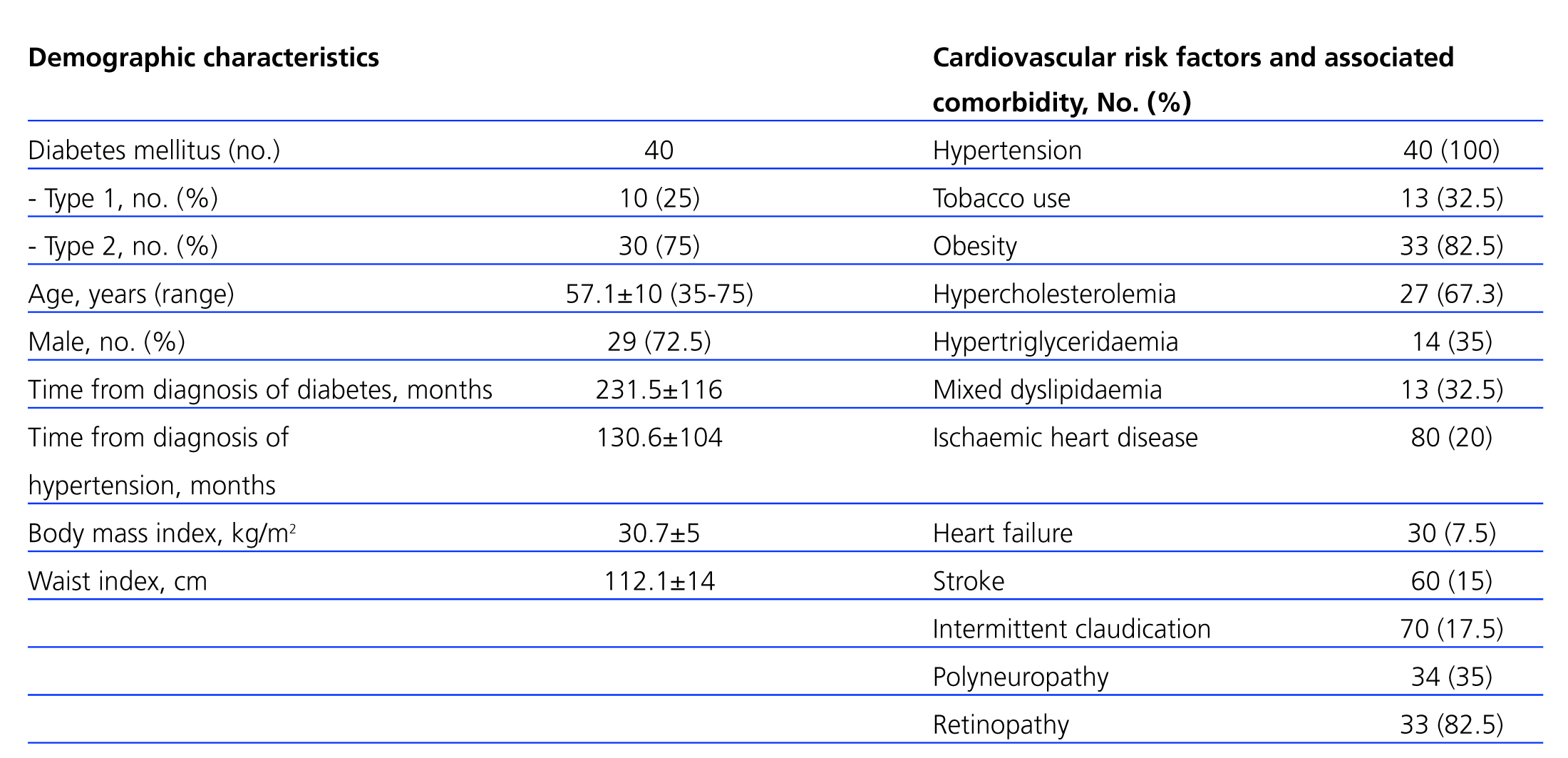
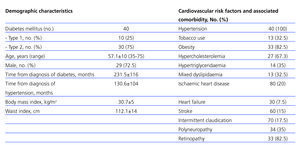
Our study included a total of 40 patients (29 [72.5%] male, with a mean age of 57.1+10 years [range: 35-76 years]). Ten patients (25%) had type 1 diabetes, and 30 (75%) had type 2 diabetes. The demographic characteristics of our patients, as well as cardiovascular risk factors and comorbidities, are summarised in Table 2.
Treatment with statins was administered to 34 patients (85%), and 33 patients (82.5%) were given anti-platelet medications. The majority of patients used insulin to control glycaemia (77.5%, 31/40), and the others used oral anti-diabetic medications (37.5%, 15/40).
Table 1 shows the anti-hypertensive medication distribution during the study period, as well as the mean number of anti-hypertensive drugs administered, which was 3.7 per patient at the end of the study, significantly higher than at baseline (n=1.8). The most commonly prescribed anti-hypertensive drugs were diuretics (n=31/77.5%), followed by alpha-beta blockers (carvedilol) (20/50%), alpha blockers (doxazosin) (17/42.5%), calcium antagonists (13/32.5%), and beta blockers (5/12%). There were no significant differences in the percentage of drug use at baseline and at the end of the study between the different types of anti-hypertensive drugs, except for alpha blockers (P<.016).
Analysis of changes in blood pressure and anthropometric parameters
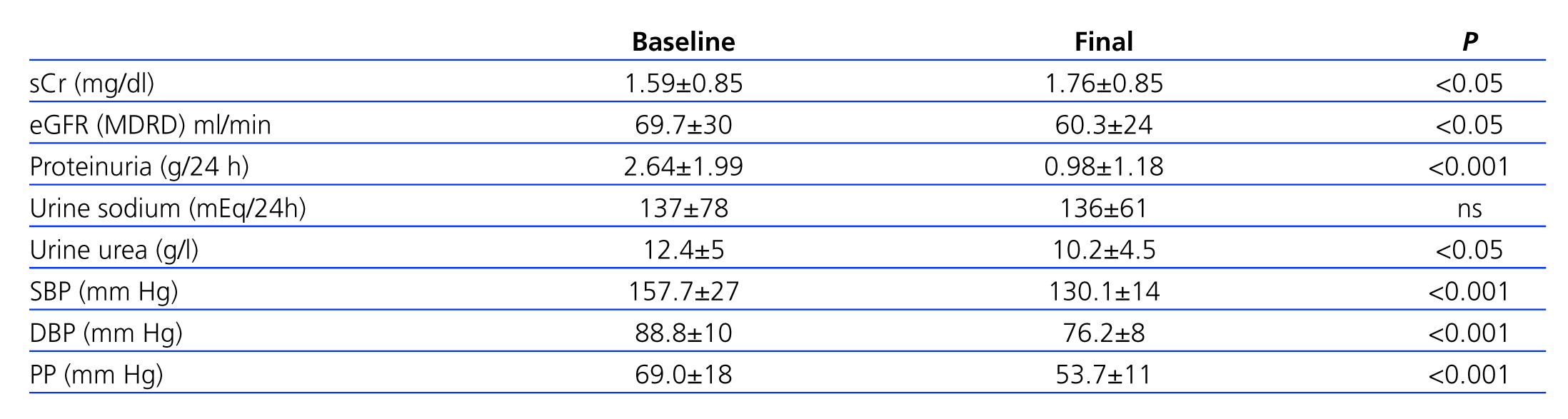
BP and PP decreased significantly from the start to the end of the study (P<.001), with a mean 27.5mm Hg for SBP (157.6±27mm Hg vs 130.1±14mm Hg), 12.6mm Hg for DBP (88.8±10mm Hg vs 76.2±8mm Hg), and 15.3mm Hg for PP (69.0±18mm Hg vs 53.7±11mm Hg). However, BMI (30.7±5.4 vs 31.1±5.5kg/m2), HR (76.9±9bpm vs 75.0±7bpm) and waist circumference (102.1±14cm vs 101.0±13cm) revealed no significant changes over the course of the study.
Analysis of biochemical parameters
Table 3 shows the changes in biochemical parameters over the course of the study period.
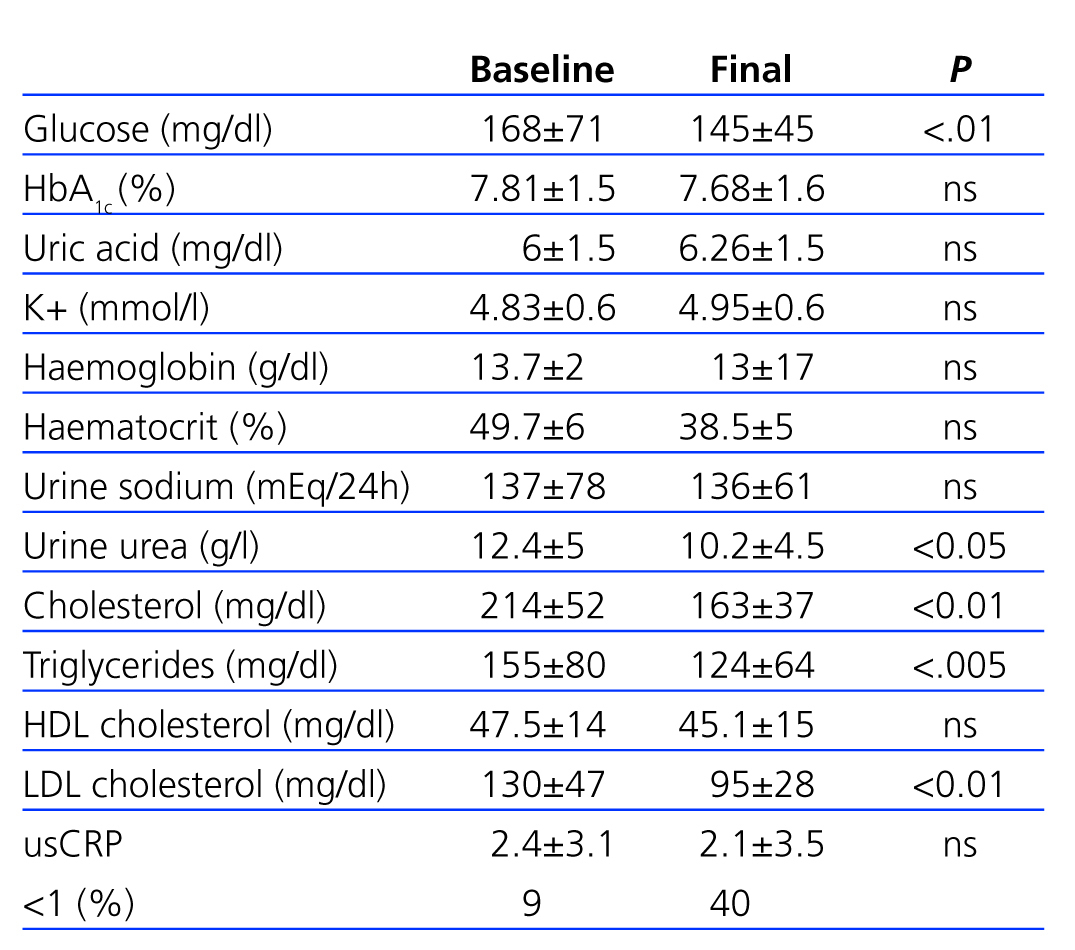
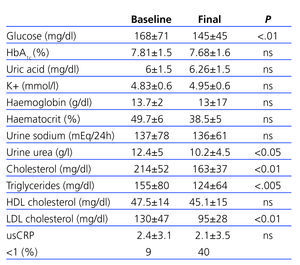
We observed no significant (ns) differences in HbA1c (7.81±1.5 vs 7.68±1.6), although there were significant changes in glycaemia (145±54 vs 168±71; P<.01). There were no significant changes in potassium (4.83±0.6mmol/l vs 4.95±0.6mmol/l), haematocrit, and haemoglobin.
Sodium urine excretion, which shows dietary intake, was stable throughout the study period (136±61mmol/24h vs 137±78mmol/24h; ns). Urine urea levels did experience a significant decrease between the start and end of the study period (12.4±5g/l vs 10.2±4.5g/l; P<.05), which suggests a reduction in protein intake.
Total cholesterol levels (214±52mg/dl vs 163±37mg/dl), LDL levels (130±47mg/dl vs 95±28mg/dl), and triglycerides (214±52mg/dl vs 163±37mg/dl) decreased significantly (P<.01); in contrast, HDL cholesterol levels did not vary significantly over the course of the study (47.4±14mg/dl vs 45.1±15mg/dl).
Analysis of the evolution of renal function and changes in proteinuria
Table 4 summarises the changes in proteinuria and renal function. We observed a significant increase in sCr (1.59±0.85mg/dl vs 1.76±0.85mg/dl; P<.05), although quantitatively, this difference was of only 0.17mg/dl. Estimated glomerular filtration rate (eGFR) decreased from 69.8±29.7ml/min/1.73m2 to 60.25±23.9ml/min/1.73m2 by the end of the third year of follow-up (3.18ml/min/year), although this rate of decrease was much lower than expected for a natural evolution of ODN (1ml/min/month =12ml/min/year).17
In patients with type 1 diabetes, eGFR decreased from 64.4±35.7ml/min/1.73m2 to 56.8±23.8ml/min/1.73m2. This difference was not significant, possibly due to the small sample size; however, it was statistically significant for patients with type 2 diabetes (71±ml/min/1.73m2 vs 61±ml/min/1.73m2; P<.05).
Over the 3-year study period, only 4 patients experienced a deterioration in urine proteinuria values (10th percentile =-4.69%), whereas values decreased by over half in more than 65% of patients, and 95% had a recovery >90%. The mean value of proteinuria before treatment was 2.64±1.99g/24h, which decreased to 0.98±1.18g/24h after the study (P<.001) (Figure 1). Normal albumin levels were reached in 25% of patients (microalbuminuria [MAU] <30mg/day).
Proteinuria values in patients with type 1 diabetes decreased from 2.06±1.18g/24h to 0.24±1.99g/24h (P<.001), whereas the decrease in type 2 patients was 2.89±2.2g/24h to 1.22±1.27g/24h (P<.001). When analysing the percent reduction in proteinuria based on type of diabetes, the effect appears to be greater in type 1 diabetes patients than type 2 diabetes patients (82.32% and 40.44%, respectively; P=.02), which could be related to the higher number of factors for progression of diabetic nephropathy in type 2 diabetes patients.
Influence of factors for the progression of diabetic nephropathy in reducing proteinuria
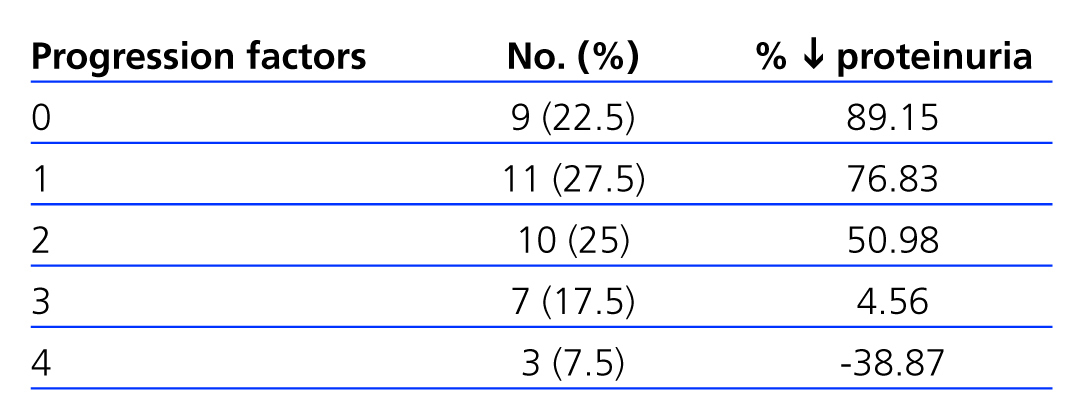
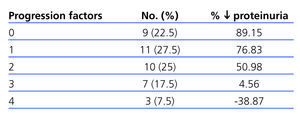
We observed a negative correlation between the number of factors of disease progression (tobacco use, BP≥140/90mm Hg, BMI≥30, proteinuria >1g/day, HbA1c≥8%, and Hb<11g/dl) and decrease in proteinuria (Table 5), with less than a 50% reduction in patients with 3 or 4 progression factors.
Analysis of the level of compliance with the therapeutic targets of the American Diabetes Association
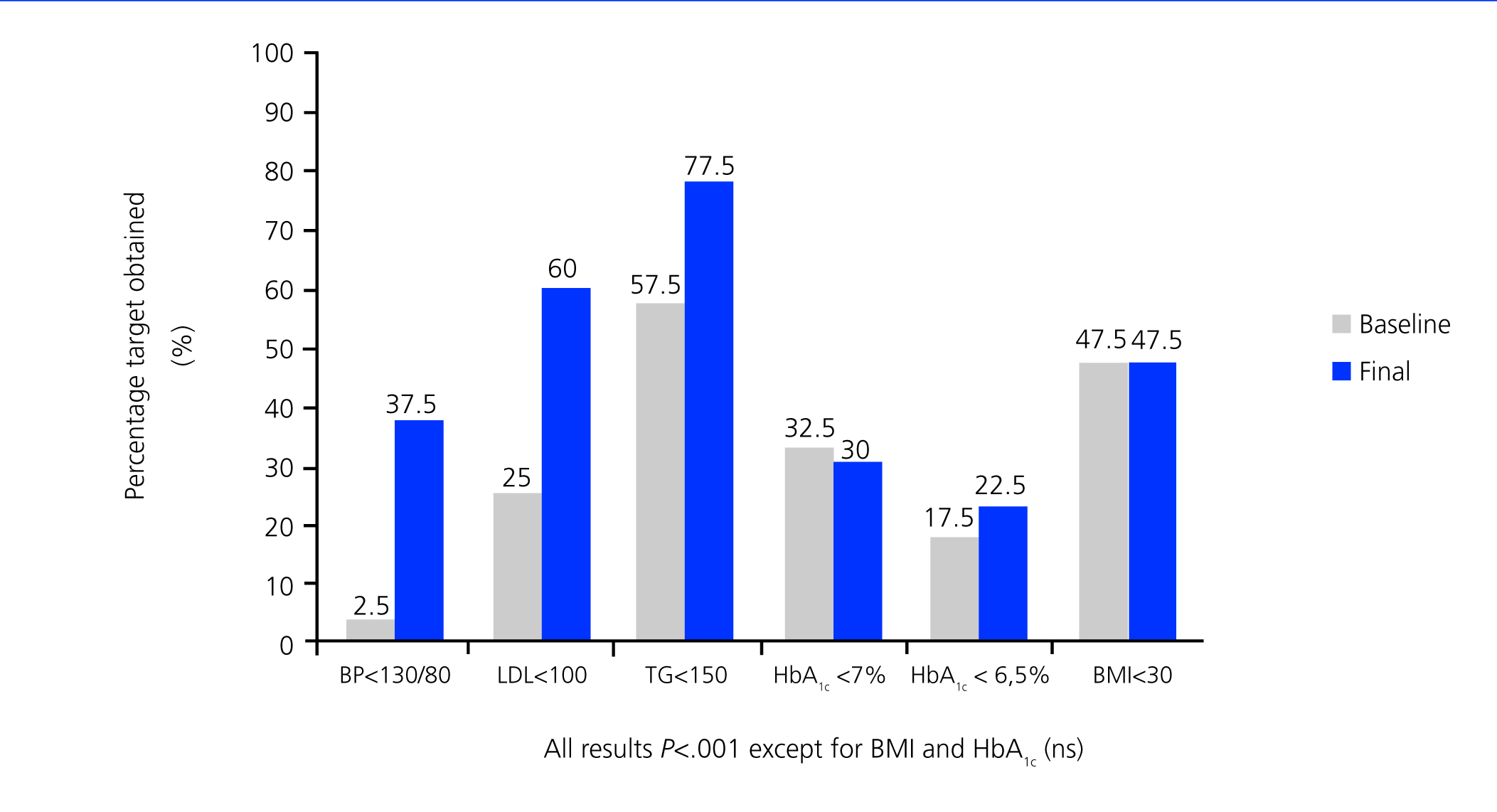
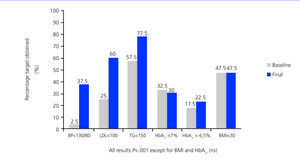
Except for HbA1c and BMI, which remained unchanged, all other variables included in the therapeutic targets set forth by the ADA3 improved significantly by the end of the study.
DISCUSSION
Our study shows that long-term multifactorial treatment based on ultra-high doses (600mg/day) of irbesartan is well tolerated and can effectively reduce proteinuria, aiding in the stabilisation of renal function in patients with ODN, which has a positive health and socio-economic impact.1,4,7
Diabetic patients that do not receive adequate medical treatment have a high risk for developing micro/macrovascular complications.18 In modern clinical practice, we are aware of a number of factors for the progression of ODN towards ACKD that must be evaluated and controlled in order to delay or halt this deterioration. Among these factors, BP stands out along with proteinuria and glycaemia as the primary risk factors, although tobacco use, anaemia, and excess weight also play an important role in this deterioration.16
A multifactorial therapeutic approach is necessary that provides the tightest possible control of all these progression factors in order to reduce the appearance and evolution of these complications.
With this multifactorial objective in mind, we established a compound therapeutic strategy for these patients that, as shown in Figure 1, allowed us to significantly increase the level of compliance with the majority of treatment targets set forth by the ADA.3 Only control of glycaemia and BMI did not improved significantly (possibly due to non-compliance with the hygienic/dietary measures proposed). These results are also in accordance with those obtained by other authors from specialised diabetic clinics.5
The need for pharmacological blockade of the RAS pathway in diabetic patients is well established, above all in the presence of microangiopathic complications.19,20
ARB have a demonstrated efficacy in controlling BP and reducing urine excretion of albumin21 in ODN patients, as well as in slowing the progression towards ACKD.8,10,21,22 However, and regardless of the adequacy of the multifactorial targets for reducing cardiovascular risk, the renoprotective results in ODN8,9 have not been so remarkable, which could in part be a consequence of the weak blockade of AT1 receptors achieved at “anti-hypertensive doses” of these drugs. In fact, two multi-centre studies involving ultra-high doses of ARB19,23 showed, in addition to a similar control of BP, a direct correlation between the dose of ARB and reduction in proteinuria, and excellent clinical/biochemical tolerance.
Our hypothesis was based on the possibility of increasing the anti-proteinuria benefits of ARB by increasing the percentage of AT1 receptors blocked with higher doses of these drugs, since the role of angiotensin II AT1 receptor is essential in mediating the intracellular renal pathological effects of angiotensin II, and so the higher the percentage of receptors blocked, the higher the benefit of this treatment.
Our treatment regimen was based on ultra-high doses of ARB,21 in accordance with those used in other studies.19,23 A dose of irbesartan 600mg in two divided doses per day (morning and afternoon) was established by modifying the plasma distribution curve until maintaining higher blood concentrations during a 24-hour period, thus reaching a higher level of activity of the drug during the 24h (these patients tend to have a non-dipper BP pattern14) and a greater blockade of the AT1 receptors during the time in bed at night. During this time, a greater positive impact on intraglomerular pressure is achieved and, in turn, a greater effect on proteinuria reduction and renal protection.
Although the majority of the patients had a proteinuria >1g/24h and the BP target in our patients would be <125/75mm Hg based on the guideline recommendations,3,14,15 this criterion is currently modified on an individual basis.15 Therefore, we added to the irbesartan-based regimen whichever anti-hypertensive drugs were necessary for achieving target BP values (Table 1). The most commonly used drugs were diuretics (thiazides or loop diuretics based on the presence/absence of renal dysfunction), followed by carvedilol and doxazosin. Although the guidelines state that calcium antagonists should be used in combination with ARB in CKD patients,14,15 they were not used heavily at the end of the study (32.5%). They were withdrawn throughout the study period because of their secondary side effects (oedema).
Following the multifactorial approach, most of patients were also prescribed anti-platelets (82.5%) and statins (85%), which contributed to the favourable changes observed in lipid profiles.
In accordance with the results available in the medical literature, many patients had type 2 diabetes,4 with higher prevalence of males and metabolic syndrome in the clinical profile (Table 2),25 as well as a high prevalence of associated risk factors3 and a high incidence of macro/microangiopathic complications (Table 2). There were also high rates of diabetic retinopathy (82.5%) and neuropathy (35%) that accompany deficient glucose control (HbA1c: 7.81±1.5%) (Table 3).
Although we observed increased mean serum creatinine values (1.59±0.85mg/dl, Table 4), the fact that almost three fourths of our study patients were males contributed to the high mean eGFR (MDRD formula) upon inclusion in the study (69.7±30ml/min/1.73m2), which is indicative of stage 2 CKD.9 Furthermore, mean proteinuria (2.64g/24h) was indicative of advanced stages of ODN.
Upon analysis of the changes produced throughout the study period, we observed that, compared with the results from other studies,5 we were unable to improve mean BMI, waist circumference (Table 2), or glycaemia (HbA1c: 7.68±1.6%; Table 3), which in our opinion was closely related to poor dietary compliance and low physical activity.
In contrast, we did achieve an excellent decrease in BP, reaching 130.1/76.2mm Hg, which definitely contributed to the decrease in proteinuria observed in our patients.3,15
We did not observe any significant changes over the course of the study in uric acid levels, haematocrit, haemoglobin, or potassium (Table 3). Despite the use of diuretics, we observed no significant changes in uricemia, which is primarily related to losartan use10 but could also be related to the uricosuric effect of ARB and the use of moderate doses of these drugs. Furthermore, it is well established that, in contrast to the effects of ACE inhibitors,25 ARB upregulate the expression of AT2 receptors and therefore do not reduce haemoglobin or haematocrit values.26 Despite the ultra-high doses of irbesartan used, and through not well defined mechanisms, K+ levels did not increased significantly, similar to the results from other studies.19,23
In the analysis of the changes produced in proteinuria and renal function (sCr, eGFR), we observed a marked global decrease in proteinuria and a tendency for stabilisation of renal function after 3 years of follow-up up to figures that has not previously been observed in other studies involving ARB treatment of ODN patients.10,20 These extremely beneficial results, with very few side effects, are very promising for daily clinical practice but need to be backed by prospective and well-designed clinical trials that include relevant clinical outcome variables such as progression towards terminal CKD and cardiovascular comorbidity. As described by other authors,11 standard anti-hypertensive doses of ARB allow for blocking approximately 35%-40% of receptors, and this value is almost doubled when using a double dose, reaching the same level of inhibition as the combination of ARB and ACE inhibitors.11,13
Although both sCr and eGFR (MDRD formula) changed significantly (P<.05) after three years, the changes in these values (Table 4) were not clinically relevant, and no patient experienced a doubling of baseline creatinine values or required dialysis. The sharpest decrease in these parameters was 24-hour proteinuria, which dropped from 2.6±1.99g/24h to 0.98±1.1g/24h (P<.001), a mean decrease of 59.2%, which is significantly higher than the results obtained in the RENAAL8 and IDNT9 studies.
At the end of the follow-up period, only 4 patients (those with more progression factors) (Table 5) had worse proteinuria values than at baseline. As the presence of progression factors for nephropathy increases, the lowering effect upon them decreases. In 75% of our patients (those with 0, 1, or 2 progression factors) (Table 5), the decrease in proteinuria exceeded 60% of the baseline values; of these, 10 (25%) achieved normal albumin levels. Overall, the decrease in proteinuria was greater in the 10 patients with type 1 diabetes, which we believe is due to the scarcity of progression factors for ODN in this group (2 smokers). On the other hand, we do not believe that the evolution of our patients was strongly influenced by the control of glycaemia levels, which did not vary significantly. These results point towards the need for an intensive multifactorial approach that facilitates reaching the recommended therapeutic targets.3,14,15
The renoprotective effects of angiotensin II AT1 receptor blockade in ODN patients appear to be primarily based on blocking the angiotensin II activity in renal tissue, since this enzyme is very active in the renal cortex of diabetic patients, where we can detect upregulation of the expression of renin and AT1 receptors.27-29
In addition to the changes in intraglomerular haemodynamics (decrease in intraglomerular pressure), the anti-proteinuria effects of ARB appear to be mediated by structural changes in the interstitial/mesangial and glomerular capillaries. The angiotensin II blockade improves the selectivity of the charge and size of glomerular membrane pores, which is in part associated with the loss of nephrin in the podocytes of the glomerular capillaries, which play a leading role in the functioning of the glomerular filtration barrier.30 Additionally, ARB seem to block other effects mediated by angiotensin II, such as endothelial dysfunction, oxidative stress, inflammation, and collagen production,31-36 which seems to be related to their anti-proteinuria effects. The benefits derived from blocking these pathophysiological mechanisms are also corroborated by indicators of regression of renal damage that have been obtained in experimental animal studies using high doses of ARB.37-39
Could we expect the same therapeutic effects with the use of ACE inhibitors as those observed with ARB?
Except for the benefits demonstrated by Lewis et al40 using captopril on diabetic nephropathy in patients with type 1 diabetes, the sparse data available regarding ODN patients with type 2 diabetes and treated with ACE inhibitors are not particularly relevant in terms of number of studies, number of patients, and follow-up periods.
In the REIN study,41 the renoprotective benefits of ramipril in diabetic nephropathy patients were very limited, and patients assigned treatment with ramipril lost renal function to a greater degree than those that were assigned other types of anti-hypertensive drugs. The ALLHAT study,42 with a mean 4.9 year follow-up period, also failed to demonstrate differences between lisinopril or amlodipine and chlorthalidone in the development of ACKD or a decrease >50% in eGFR. Even so, we must take into account that the ALLHAT study was not designed to evaluate renal function, since it did not register information regarding baseline or post-treatment proteinuria/albuminuria, or other aspects related to renal failure. On the other hand, Suissa et al43 performed a comparative study using diuretic treatment, and observed an increase (up to 2.5-fold) in the risk of developing ACKD in diabetic patients treated with ACE inhibitors. Although the authors did not explain these results, the fact that the patients treated with these drugs were at a high risk for developing ACKD may have had some influence on them.
In contrast, one study that analysed the evolution of MAU in patients with incipient nephropathy and type 2 diabetes (in earlier stages of diabetic nephropathy) showed that ACE inhibitors did indeed slow the progression towards ODN.44
This lack of conclusive results in studies involving the treatment of ODN using ACE inhibitors could be related to the pathophysiological and pharmacological differences between ACE inhibitors and ARB in terms of the renal effects of angiotensin II.
The strength of the angiotensin II blockade that is achieved using ARB, by selectively acting upon AT1 receptors, could endow these drugs with a greater renoprotective benefit than ACE inhibitors. In addition to selectivity, the blockade of the AT1 receptor with ARB is more intense and longer lasting than with ACE inhibitors. Additionally, this selective inhibition of the AT1 receptor reduces the cellular uptake of angiotensin II and its intracellular effects, including the capacity to activate the expression of new AT1 receptors in the cellular membrane through a positive feedback loop, thus favouring an even higher rate of angiotensin II uptake.
Another difference between ACE inhibitors and ARB is the fact that, in diabetic nephropathy patients, the majority of angiotensin II molecules are not generated through the converting enzyme pathway, but rather through an alternate pathway involving the activation of chymase.27,45 In this manner, in diabetic nephropathy, renal tissue is infiltrated by monocytes that release chymase, which activates the direct conversion from angiotensin I to angiotensin II within the kidney.45
But perhaps the most relevant difference between ACE inhibitors and ARB that would explain their different levels of renoprotection is that ARB upregulate the expression of AT2 receptors through a bio-feedback mechanism together with the selective blockade of the AT1 receptor, which is not observed with ACE inhibitors. In addition to contributing to the haemodynamic impact by reducing BP, there is increasing evidence of the renoprotective effects of the activation of the AT2 receptor by ARB, thus participating in the self-regulation of renal blood flow (especially in cases of low cardiac output) and the renal structural changes that aid in slowing the progression of ODN towards ACKD.25,29,46
The differences in the clinical management of diabetic chronic renal patients must also be considered. On the one hand, thanks to the upregulation of AT2 receptors, these drugs appear to have a milder impact on haematocrit and haemoglobin levels,25 as observed in our patients. On the other hand, they also provide a better clinical and biochemical tolerance, with very few patients experiencing severe hyperkalemia (K+>6mmol/l) or requiring treatment suspension, in contrast to the results observed when using ACE inhibitors alone or in combination with ARB.47 A recent post-hoc analysis of the ONTARGET study48 also failed to show renoprotective benefits in this combination in patients with a high risk of chronic renal failure or proteinuria. In our study, no patients had K+ levels higher than 5.5mmol/l, which supports the findings from other studies47,48 where the rate of hyperkalemia also was low.
We analysed the inflammation grade by monitoring serum levels by ultra-sensitive CRP in order to evaluate the possible influence of ARB on this value. However, although CRP levels did decrease slightly, this difference was not statistically significant (2.4±3.1mg baseline vs 2.1±3.5mg at the end of the study). The lipid profile improved slightly, essentially due to treatment with statins, although it is possible that the high doses of irbesartan contributed to this result, as the AT1 receptor blockade has beneficial effects on insulin resistance.49,50
Our study did have certain limitations. Firstly, it was not a randomised study, and our sample size was small, although the consistency observed in our results made up for this negative factor. Secondly, it was not designed to analyse cardiovascular morbidity or mortality; however, previous studies found no difference in the incidence of cardiovascular events,5,10 and these findings could be extrapolated to our own study. Thirdly, we did not maintain a control group on irbesartan 300mg in order to compare our results with the renoprotective effects of a reduced dosage, although the results from the IDNT study,9,20 using irbesartan at 300mg, were less significant than ours. Even so, randomised studies will be needed that compare these ultra-high doses to conventional doses of ARB. Apart from to the economic implications of performing a large study with these characteristics, an analysis of the rationale used to support high doses of ARB in these patients and the results obtained in daily clinical practice would probably support the use of high doses.
In conclusion, a multifactorial treatment of ODN based on irbesartan 600mg daily is a highly effective and safe method for reducing proteinuria and slowing the evolution towards terminal chronic renal failure, making this a good therapeutic alternative for these patients.
Conflicts of Interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Treatments administered over the course of the study period
Table 2. Demographic characteristics, cardiovascular risk factors, and comorbidities associated with the study patients
Table 3. Evolution of biochemical parameters over the course of the study period
Table 4. Comparative differences in terms of renal parameters and blood pressure values
Table 5. Association between factors for the progression of chronic renal failure and reduction in proteinuria
Figure 2. Compliance with the objectives established by ADA recommendations
10962_19157_27965_en_w4777121925ref.1096212172_10962_18725_16923_es_figura_1_en.doc
Figure 1. Evolution of proteinuria over the study period