Se presenta el caso de un paciente con un diagnóstico de probabilidad de trombocitopenia inmune inducida por heparina (TIH II) con base en criterios clínicos y test de ELISA positivo, en que la urgencia terapéutica fue determinante ante la gravedad del proceso. La suspensión de heparina y la administración de argatroban solventaron la ineficacia de la diálisis debida a coagulación repetida del circuito y catéter, permitiendo el paso a diálisis peritoneal sin mayores contratiempos. Consideramos prioritaria la toma de decisiones antes de certificar la seguridad diagnóstica.
We present the case of a patient diagnosed with likely immune heparin-induced thrombocytopenia (HIT II) based on clinical criteria and a positive ELISA test, in which emergency treatment was crucial, given the seriousness of the process. The discontinuation of heparin and administration of argatroban resolved inefficiency of dialysis resulting from repeated coagulation of the circuit and catheter, allowing peritoneal dialysis without further setbacks. We consider decision-making to be a priority before certifying diagnostic confidence.
INTRODUCTION
Heparin-induced thrombocytopenia (HIT) in haemodialysis may be of two types: type I (HIT I), which is mild, self-limiting, it appears 1-4 days after administration and has an incidence of 10%-20%; the second type (HIT II) is severe, it appears between 5 and 15 days after the first exposure to heparin and it results in a decrease in the number of platelets greater than 30%-50% on the baseline value, with a platelet count that is generally lower than 100x10/l1. It is immune and may be fatal, due to often being accompanied by thromboembolic events, such as heart attacks, skin necrosis, pulmonary embolism and gangrene. Its incidence is around 1%-2%.
The pathogeny consists of antibody formation, generally IgG, against the antigen complex consisting of the heparin platelet factor 4 complex with platelet activation and uncontrolled thrombin activation. Its firm diagnosis requires functional tests to detect antibodies against the aforementioned antigen2. The treatment must be immediate, since mortality of up to 30% has been reported3.
We treated a case with characteristics typical of the process as appearing in the event probability scale (4T)4 (Table 1), and we decided to carry out empirical treatment before receiving the results of the ELISA (enzyme-linked immunosorbent assay), since functional tests were not available. At that time, we reviewed all forms of heparinisation and sterilisation of the circuit, without realising that the catheter manufacture report included ethylene oxide use for its sterilisation, as well as its impregnation with heparin. The choice of argatroban was based on its suitable pharmacokinetic profile in renal failure and in haemodialysis5.
CASE REPORT
71-year-old patient diagnosed with advanced chronic renal failure secondary to nephroangiosclerosis; it was advisable to start haemodialysis due to progressive asthenia and a glomerular filtration rate <10ml/min. The patient received treatment with acenocoumarol, due to a history of atrial fibrillation and ischaemic stroke. It was not possible to create an arteriovenous fistula due to poor arterial and venous development in the arms.
Three days before the first dialysis session, low-molecular-weight heparin administration was initiated to replace the patient’s regular anticoagulant. A left jugular tunnelled catheter was inserted, with regular doses of heparin administered to prevent coagulation of the circuit, as well as unfractionated heparin for catheter sealing. The platelet count was 210x109/l. The first sessions were unsatisfactory due to poor catheter performance. Nine days after the first administration of heparin, in the fourth dialysis session, we treated the first acute event: sudden dyspnoea and chest pain at the start of the session without low blood pressure, haemoptysis or fever. Blood pressure: 180/100mmHg, tachypnoea, no fever, jugular vein engorgement or cyanosis, with a heart rate of 90 beats per minute, with systolic ejection murmur II/VI, without ectopic beats or pericardial rubs, bronchospasms without crepitant rales, no oedema or signs of deep venous thrombosis, with normal foot temperature and colouring. No skin rash. Haemoglobin: 9.6g/dl, leukocytes: 7200. Platelets: 44x109/l (Figure 1), lactate dehydrogenase (LDH): 510U/l, cardiac troponin 1 (cTnI): 2.6ng/ml. No signs of ischaemia or lesions in the electrocardiogram. The patient was observed for 24 hours with normalisation of clinical profile (over the course of the session) and with a significant decrease in the cTnI figure to 1.2ng/ml. The x-ray did not provide relevant data.
The filter used was made of steam-sterilised polysulfone. Haemodialysis was conventional with volume control. The patient did not receive therapy with renin-angiotensin system blockers, but rather with beta-blockers and calcium channel blockers. At that time, we did not realise that the catheter manufacturing information specified sterilisation with ethylene oxide and its impregnation with heparin.
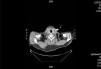
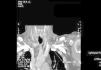
We decided to discontinue heparin due to a suspicion of HIT II, with the following sessions experiencing many catheter flow and resistance problems aside from repeated circuit coagulations. While awaiting treatment with argatroban, the jugular tunnelled catheter was replaced by another with similar characteristics, without improved performance, and as such, a cervical computerised axial tomography scan was taken, and we observed partial thrombosis of the left jugular vein (Figures 2 and 3). Two days later, the patient presented at the unit without symptoms, but 10 minutes after being connected, they experienced a new episode of sudden dyspnoea with p02 of 78%, from which they recovered in 1 hour with the normal support measures and did not require hospital admission. The platelet count was 101x109/l, which was below that of the previous days. Forty-eight hours later the platelets were 162x109/l. Finally, argatroban became available and was administered at the previously reported bolus dose of 2590mic/kg at the start of the session and at the subsequent perfusion dose of 2mic/kg/min, which finished 1 hour before disconnection. The activated partial thromboplastin time (APTT) controls were maintained within the recommended range of 1.5 to 2.5 over the baseline value. After that, circuit coagulations disappeared and the performance of the same catheter was excellent. During the waiting period before starting treatment with the thrombin inhibitor, and afterwards, until the patient finally started peritoneal dialysis, antiaggregant treatment was prescribed in the form of clopidogrel. This was due to the threat of new central vessel punctures and two pending surgical interventions: peritoneal catheter and transurethral resection of a small bladder tumour, although the latter was carried out when peritoneal dialysis had already begun. At no time before the patient was transferred to the reference hospital for a change of renal replacement therapy were argatroban and dicoumarins administered together. We must also mention the absence of acute venous thrombosis data and that the heart rate was controlled.
Clinical and laboratory data
HIT II is defined as a 50% decrease in the number of platelets, with a count below 100x109/l, which occurs within between 5 and 15 days of the first exposure. In our setting, this condition has not gone unnoticed 6-8. The considerable risk of thrombosis means that we must act quickly. The diagnosis is carried out by functional and antigen tests. The first, more specifically STRA (14C serotonin release assay), HIPA (heparin-induced platelet activation assay) and PAT (platelet aggregation test)9,10, have the disadvantage of not being available in all hospitals, with STRA being the main functional test due to its specificity (99%). The antigen tests using ELISA and the PaGIA (particle gel immuno assay) do not achieve this degree of specificity11.
Diagnosis
The clinical process permits other assumptions: an allergic reaction to ethylene oxide12 usually manifests with a skin reaction and does not explain thrombocytopenia or the rapid recovery without anti-histamines. Ethylene oxide antibodies were not determined. Another condition with a similar clinical presentation is the anaphylactoid reaction upon using negatively charged filters in patients treated with renin-angiotensin system blockers due to bradykinin activation13,14, and the patient was not taking these drugs. Other contaminants or sterilisers in the circuit seem very unlikely in haemodialysis with the current control systems. The scheduled determination of endotoxins in the dialysate was always normal.
In extracorporeal treatment, it is necessary to rule out air embolisms, haemolysis and acute anaemia. The visible area of the catheter was intact and no leaks were detected in the circuit. There were no haemolysis data, with LDH of 501U/l.
Situations inherent to patient pathology, such as pulmonary oedema, heart attack, embolism or pericardial tamponade, can be excluded in the clinical, biological and radiological context. It is true that there was a certain amount of myocardial suffering in view of the increased cTnI figure, but there were no electrocardiographic changes. The possibility of sepsis seems unlikely without a fever or leukocytes, and as such, procalcitonin was not determined.
Thrombocytopenia was not explained by the patient’s normal pharmacology, or by pulmonary sequestration with biocompatible filters, sepsis or disseminated intravascular coagulation (DIC), with it being confirmed by the Haematology department that it was not a spurious form.
Bearing in mind the chronopathology of the event and following the parameters of the test mentioned (Table 1) with a subjective score of 7, we decided to adjust to a presumptive diagnosis of HIT II, later supported by a positive ELISA test (Table 2), weighing up the risks and benefits.
Treatment
We should not wait for diagnostic confirmation from laboratory tests when there is strong clinical suspicion. The first step is to discontinue heparin. The drugs indicated are thrombin inhibitors: lepirudin, bivalirudin and argatroban. The first has the disadvantage of causing anaphylaxis and it is excreted by the kidneys. Although it is not currently available, it has been successfully used in our setting6. Bivalirudin use is generalised in catheterisation laboratories due to its short half-life, however, it is also excreted by the kidneys15. Argatroban is not excreted by the kidneys and it therefore seems more suitable in Nephrology; it is acquired as foreign drug.
Argatroban16 is a synthetic thrombin inhibitor, to which it binds selectively and reversibly. It has a short half-life, and as such, its effect disappears soon after it is discontinued. It is not immunogenic and does not require dose modification in renal failure. It is capable of inhibiting both free thrombin and clot-bound thrombin. It is metabolised in the liver and eliminated in bile and finally in faeces. Equilibrium concentrations are achieved within between 1 and 3 hours and its effect disappears 1-2 after it is discontinued. Liver and multiorgan failure require a dose reduction (0.5mic/kg/min): a quarter of the normal dose of 2mic/kg/min. Two prospective studies tested its efficacy and safety3,17 in terms of its anti-thrombotic power, without an increase in bleeding. Trials carried out in haemodialysis18-20 and haemofiltration21 demonstrated elimination of up to 20%, which should be borne in mind, since there is no antidote to the drug.
The often necessary transition from argatroban to dicoumarins must be carried out carefully, due to the risk of necrosis, gangrene and leg amputation brought on by the effect of dicoumarins in reducing protein S and C levels22,23. Thrombin inhibitors change the international normalized ratio (INR) values due to their high osmotic concentration. In practice, once platelet count is normal, argatroban can be administered alongside acenocoumarol over a minimum of 4-5 days until an INR>4 Is achieved. Then argatroban is discontinued and a new INR test is carried out after 6 hours. It was found that during the joint administration of both drugs with an INR>4, the relationship between the INR and the risk of bleeding changed, with the risk of thrombosis being higher. If a patient is taking a dicoumarin anticoagulant and HIT II is observed, vitamin K should be administered in order to reverse a greater risk of thrombosis in this context.
CONCLUSION
This case warns us about HIT II, which can often only be detected by thrombocytopenia in the time interval mentioned, after the first administration of heparin. In our case, the discontinuation of heparin and the administration of argatroban were safe and effective, and we achieved adequate dialysis as we waited for the patient to be transferred to the reference hospital for peritoneal dialysis, where they are continuing this treatment without any incidents. In the differential diagnosis, we must consider all the details, which we did not do in our case because we forgot to check the manufacture information on methods of sterilisation and impregnation of the catheter with heparin.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. 4T scoring systems for suspected heparin-induced thrombocytopenia
Table 2. Diagnostic probability of heparin-induced thrombocytopenia in haemodialysis (three clinical factors and ELISA)7
Figure 1. Platelet count in relation to time and anticoagulant treatment.
Figure 2. Jugular vein thrombosis (cross-sectional view).
Figure 3. Jugular vein thrombosis (front view).